Classification and Grouping of Laser Medical Devices in Colombia

The incorporation of laser technology to medical devices has come to play a crucial role in a diverse range of healthcare applications. Beyond their association with cosmetic procedures, many medical devices include lasers, transforming patient care and attention. From breaking down kidney stones to corneal correction, laser technology enhances healthcare and continues to evolve as […]
Classification and Grouping of Glucometers in Colombia
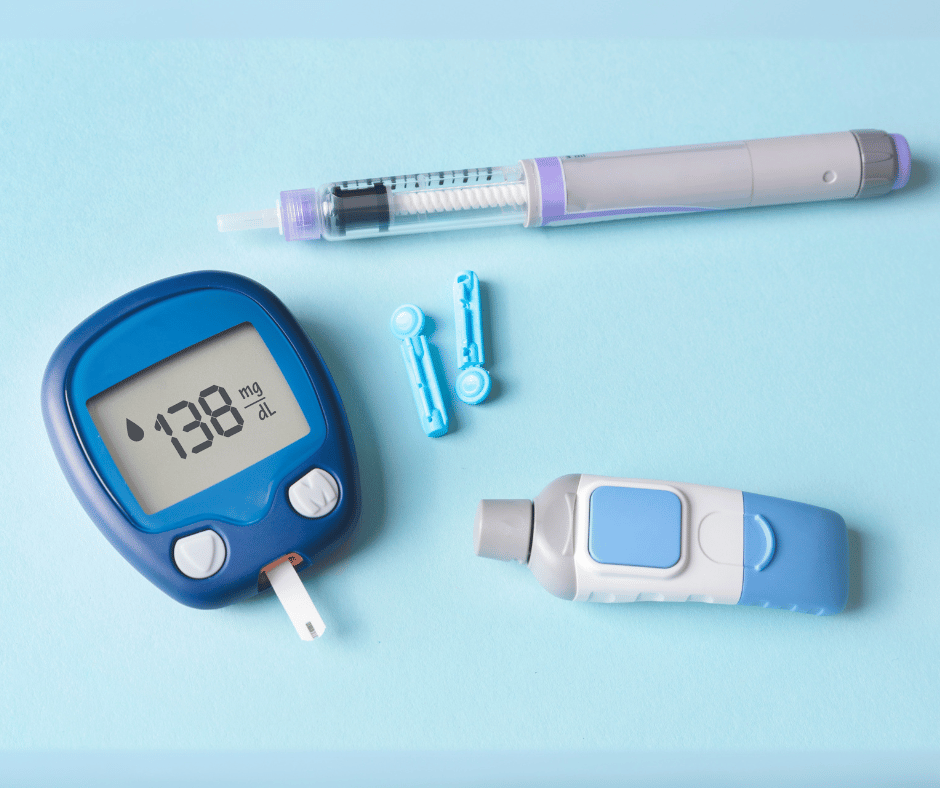
Glucometers are essential devices for individuals suffering from diabetes. Part of a diverse array of devices that help diabetic people to improve their quality of life, these compact devices provide quick and accurate readings, enabling users to make informed decisions about their diet, medication and overall health management. This article will discuss the characteristics […]
Classification and Grouping of Endoscopes in Colombia

Endoscopes are indispensable tools in modern medicine, allowing healthcare professionals to diagnose and treat conditions within the body with minimal invasiveness. With the recent advancements in medical technology, the range of endoscopic equipment has expanded significantly. This article provides an overview of the types of endoscopes, their applications, and the regulatory requirements for their classification […]
Classification and Grouping of face masks in Colombia

Face masks consist of a protective covering for the mouth and nose. They are usually made from non-woven fabric and designed to avoid spreading infectious microorganisms, such as viruses and bacteria. In regulatory terms, there are different types of face masks that also differ in their classification. In Colombia, we can define three classifications […]
Classification and Grouping of Hospital Sterilizers in Colombia

In hospitals and medical facilities, ensuring the cleanliness and sterility of medical instruments and equipment is essential to patient safety and infection control. By applying the method known as sterilization, equipment is rid of all forms of microbial life, including bacteria, viruses, fungi, and spores. In this article, we will focus on the characteristics and […]
Classification and Grouping of Dental Resins in Colombia
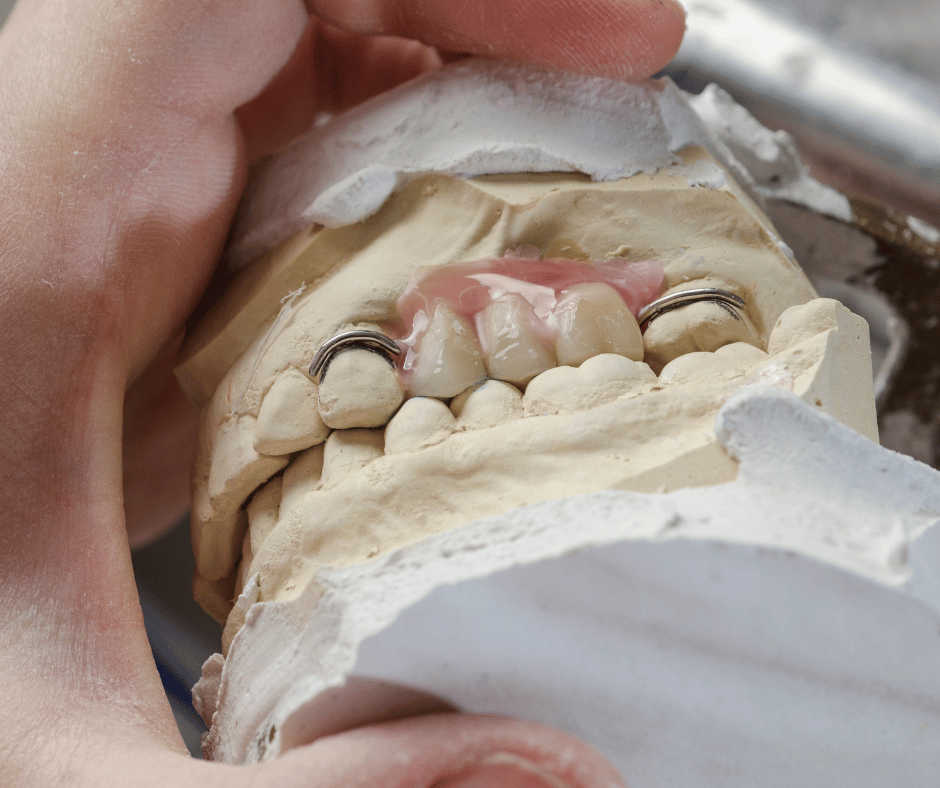
In our Classification and Grouping Ultimate Guide, we have explained how to classify and group medical devices in Colombia according to the 18 rules detailed in Decree 4725 of 2005. Also, we have explained how to make use of those rules to classify and group specific medical devices such as gloves, orthopedic implants, ultrasound systems , […]
Classification and Grouping of Defibrillators in Colombia
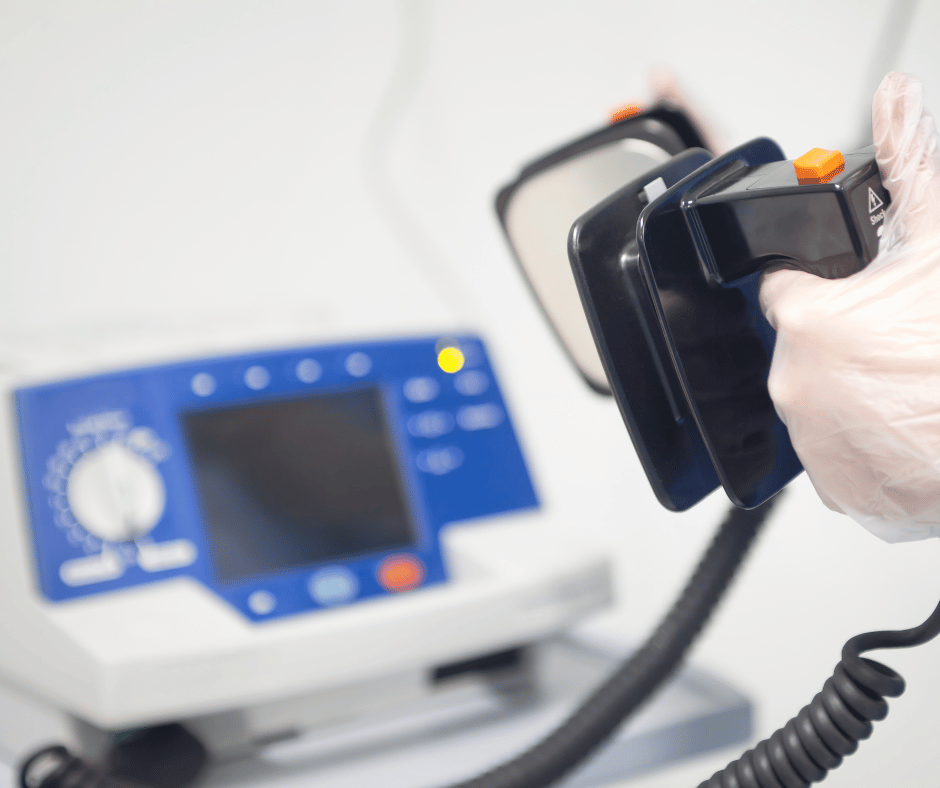
Defibrillators are considered a critical tool, not only for medical practitioners, but for anyone attempting to save a life from a cardiac arrest episode. The use of defibrillators is a crucial part of the chain of survival in cases of sudden cardiac arrest. Early defibrillation, along with cardiopulmonary resuscitation (CPR), can significantly increase the chances of survival […]
Classification and Grouping of Colposcopes in Colombia

Colposcopes are indispensable tools in gynecological examinations, significantly contributing to the early detection and treatment of cervical and other genital abnormalities. This medical device is crucial for diagnosing various conditions, including cervical cancer and other abnormalities. In this article, we analyze the components and applications of colposcopes, as well as provide general criteria for their […]
Classification and Grouping for Dialysis machines in Colombia
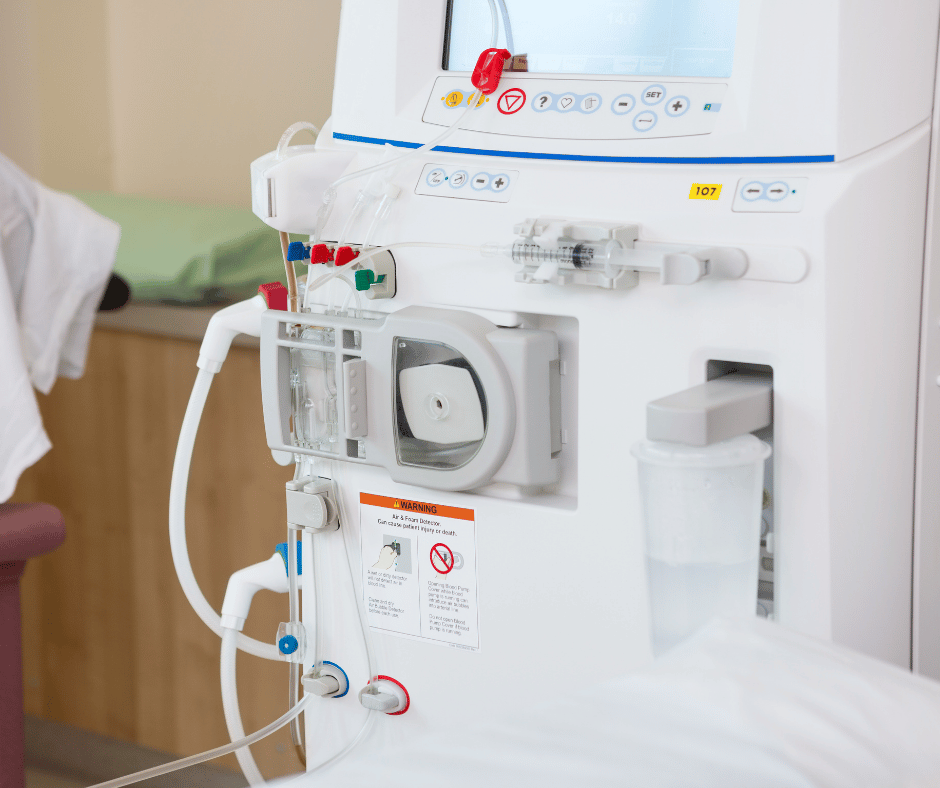
As mentioned in our Device Classification and Grouping Guide, there are a series of rules to consider when classifying and grouping medical devices. The above-mentioned rules are fully explained in Decree 4725 of 2005. In this article we will focus on explaining strategies to classify and group a Dialysis machine and the common accessories related […]
Classification and Grouping for Urology Devices in Colombia
When registering Medical Devices in Colombia, the classification and grouping strategy is a key activity for optimizing the number of registrations (based on 18 rules). You can find some examples of the classification and grouping criteria in our previous articles for: Orthopedic Implants Ultrasound Systems Medical Imaging In vitro Diagnostics Surgical instruments In […]
