Classification of Patient Monitoring Systems in Colombia
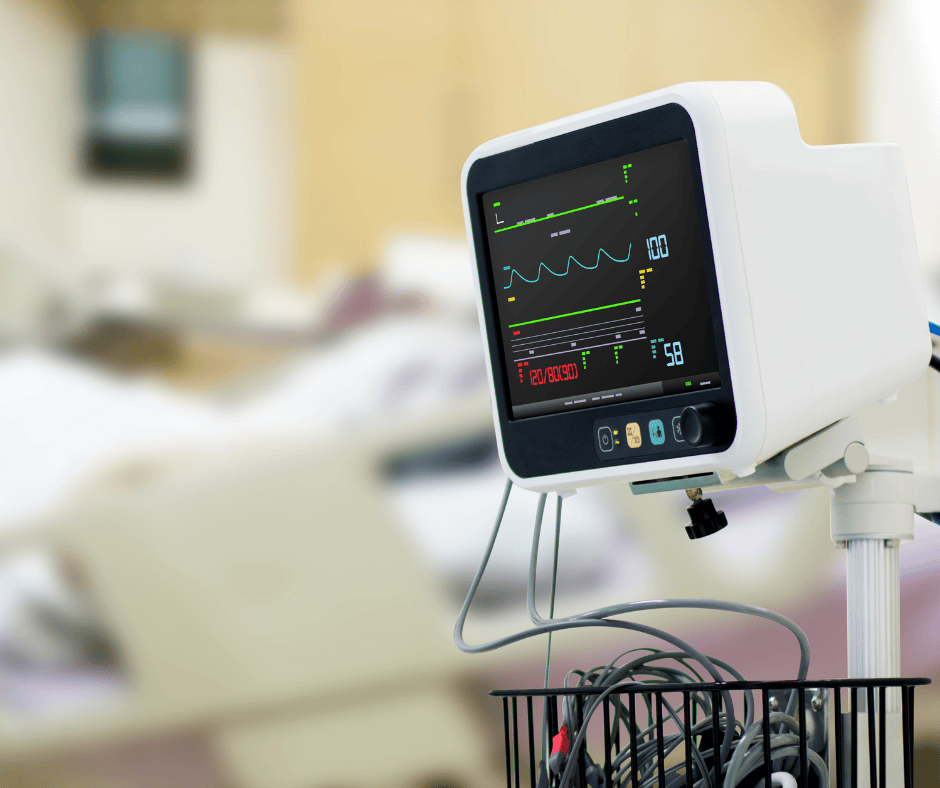
Patient monitoring systems nowadays offer an ample array of healthcare solutions. From clinical healthcare to personal use, healthcare monitors evolve to satisfy new needs, and so do their basic structure and components. This continuous state of evolution often constitutes a challenge when trying to classify and group them in the context of the Colombian regulatory […]
Classification and Grouping of Pacemakers in Colombia

Pacemakers have been considered an important precursor of many developments in science and cardiac medicine, playing a unique role in managing heart rhythm disorders. These medical devices can greatly improve the quality of life for individuals with heart rhythm disorders by reducing symptoms such as fatigue, dizziness, and fainting. They also help prevent serious complications associated […]
Classification and Grouping of CPAPs Devices in Colombia

As mentioned in our Classification and Grouping Ultimate Guide, there are a series of rules to classify and group medical devices. The above-mentioned rules are fully explained in Decree 4725 of 2005. In this article we will focus on explaining strategies to classify and group CPAP’s systems (Continuous Positive Air Pressure) and equivalent equipment […]
Classification and Grouping of Liposuction equipment in Colombia
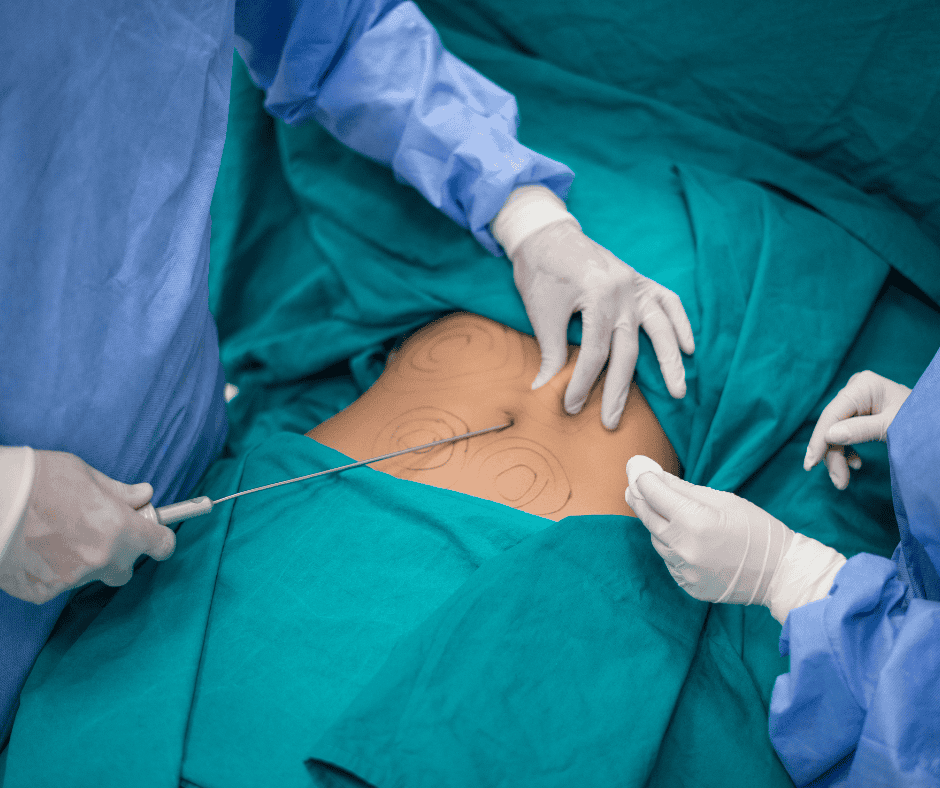
Liposuction, also known as lipoplasty, is a surgical procedure designed to remove excess fat deposits from specific areas of the body, such as the abdomen, hips, thighs, buttocks, arms, and neck. This procedure not only enhances body contour but also contributes to overall aesthetic improvement. The success and safety of liposuction largely depends on the […]
Classification and Grouping of Laryngoscopes in Colombia
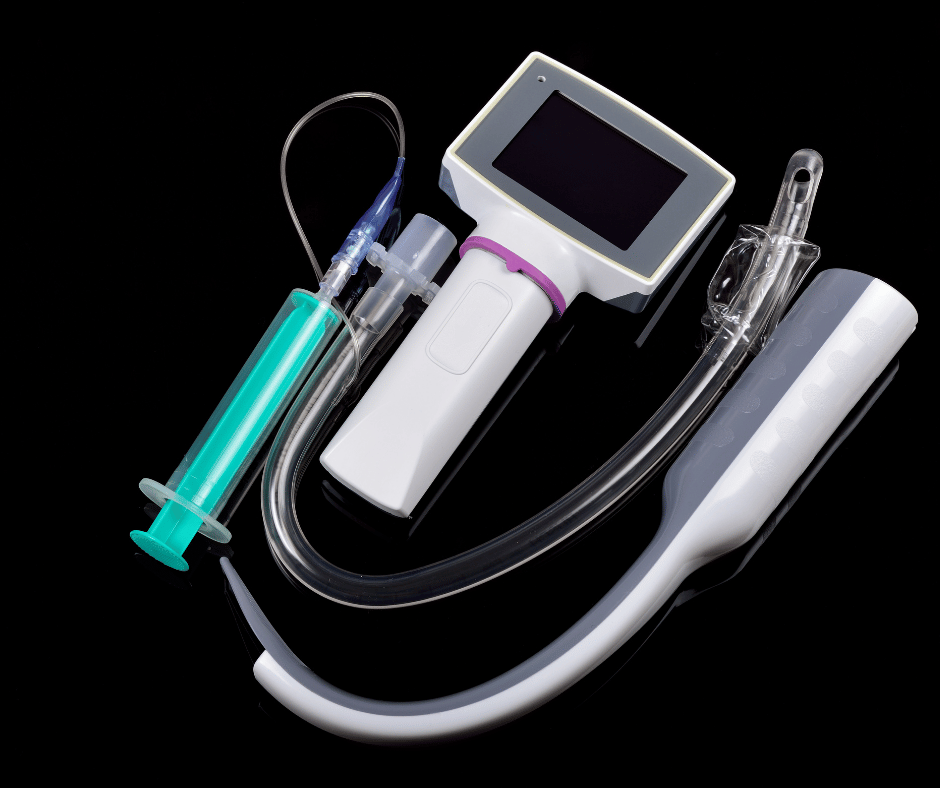
Laryngoscopes are essential medical devices used in anesthesiology and airway management. While traditional laryngoscopes have been in use for many years, advancements in technology have led to the development of video laryngoscopes. These innovative devices involve additional considerations for their sanitary requirements in Colombia. Types of Laryngoscopes Classic or Conventional Laryngoscopes: consist of […]
Classification and Regulation of Sanitizers in Colombia

Sanitizers in regulatory terms are complex products. In some countries, they are classified as cleaning products, cosmetics or biocides. In addition, their sanitary authorization is not required in all countries. This is not the case for Colombia, in which sanitizers can be classified as medical devices or require to comply with Colombian Norms. In this […]
Classification and Grouping of Laser Medical Devices in Colombia

The incorporation of laser technology to medical devices has come to play a crucial role in a diverse range of healthcare applications. Beyond their association with cosmetic procedures, many medical devices include lasers, transforming patient care and attention. From breaking down kidney stones to corneal correction, laser technology enhances healthcare and continues to evolve as […]
Classification and Grouping of Glucometers in Colombia
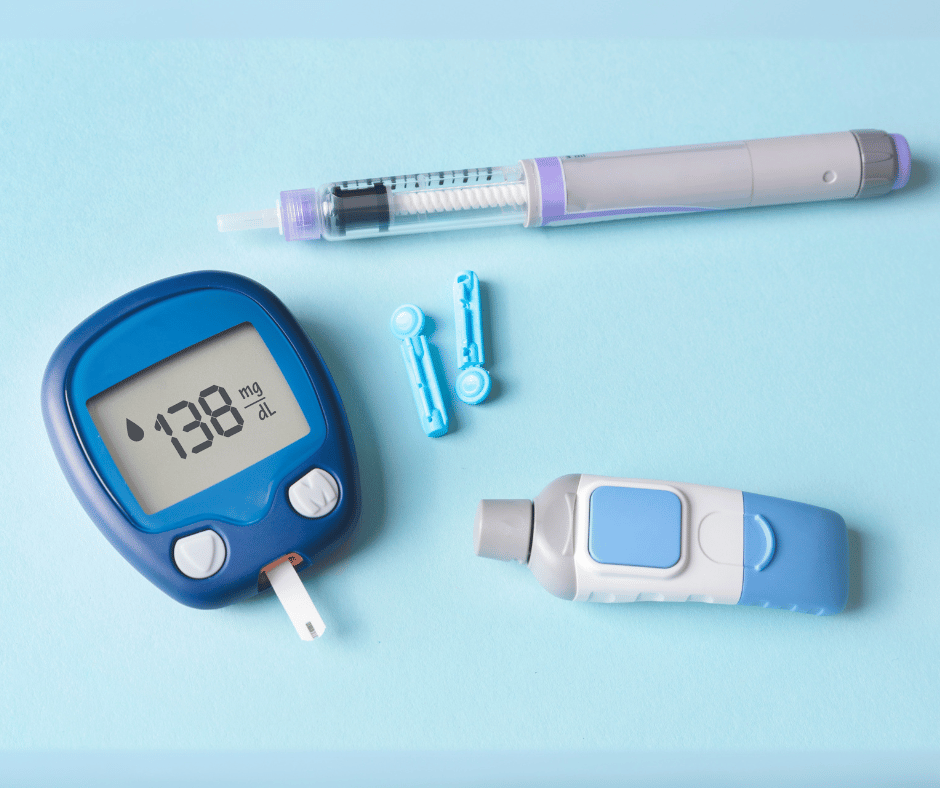
Glucometers are essential devices for individuals suffering from diabetes. Part of a diverse array of devices that help diabetic people to improve their quality of life, these compact devices provide quick and accurate readings, enabling users to make informed decisions about their diet, medication and overall health management. This article will discuss the characteristics […]
Classification and Grouping of Endoscopes in Colombia

Endoscopes are indispensable tools in modern medicine, allowing healthcare professionals to diagnose and treat conditions within the body with minimal invasiveness. With the recent advancements in medical technology, the range of endoscopic equipment has expanded significantly. This article provides an overview of the types of endoscopes, their applications, and the regulatory requirements for their classification […]
Classification and Grouping of face masks in Colombia

Face masks consist of a protective covering for the mouth and nose. They are usually made from non-woven fabric and designed to avoid spreading infectious microorganisms, such as viruses and bacteria. In regulatory terms, there are different types of face masks that also differ in their classification. In Colombia, we can define three classifications […]
