As mentioned in our Classification and Grouping Guide, there are a series of rules to consider when classifying and grouping medical devices. The above-mentioned rules are fully explained in Article No. 5 of the decree 4725 of 2005.
In this publication, we will focus on explaining strategies to classify and group an ultrasound equipment (portable and non-portable) following simple general rules and explaining some particularities that can be found according to our everyday experience.
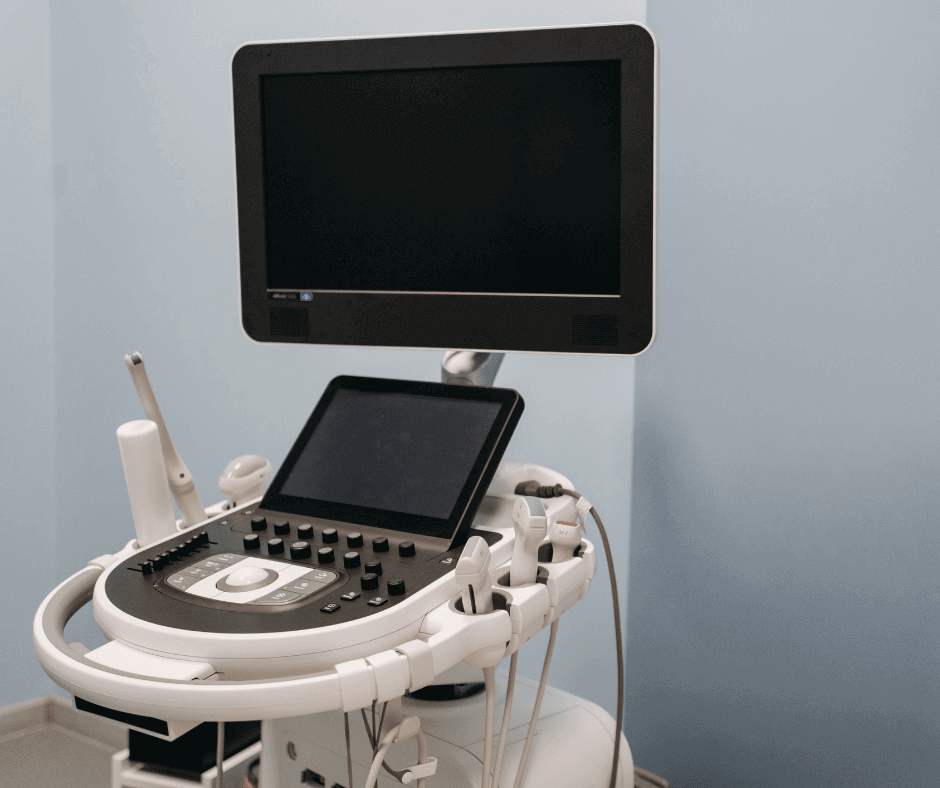
General characteristics of an Ultrasound system
Most ultrasound systems are sold with a set of transducers that get in contact with the patient. These transducers are not generally sold sterilized and they vary in size depending the type of image the user needs to take or the anatomical site the transducer was manufactured for.
On the other hand, ultrasound manufactures tend to sell these machines in different models. Most of the main differences are related to algorithms or the different tasks the system can do. Another key variation is the transducers each model can work with.
Finally, we have seen a trend in the market for portable ultrasound devices. These products basically consist of a single piece that includes the transducer, processor and a cable or wireless interface to display the image in a mobile, tablet or any other digital screen.
Classification according to the level of risk
Ultrasound equipment by its nature are classified as equipment intended for diagnosis and therapeutic devices. According to their level of risk are generally classified as class IIA – based on Chapter III classification of medical devices. The same is applicable for portable versions.
Grouping for Sanitary Registration purposes
The classification for an ultrasound system is quite straight forward in Colombia.
Regarding the grouping of accessories and different equipment, it is not necessary to take into consideration aspects such as the invasiveness of the transducers, brand or material to obtain the sanitary registration. In other words, ultrasounds systems and transducer can be grouped in the same registration, it doesn’t matter if the transducer is invasive or non-invasive.
Thanks for reading this article, if you have any question about this topic, feel free to contact us at contact@veraqueconsulting.com.