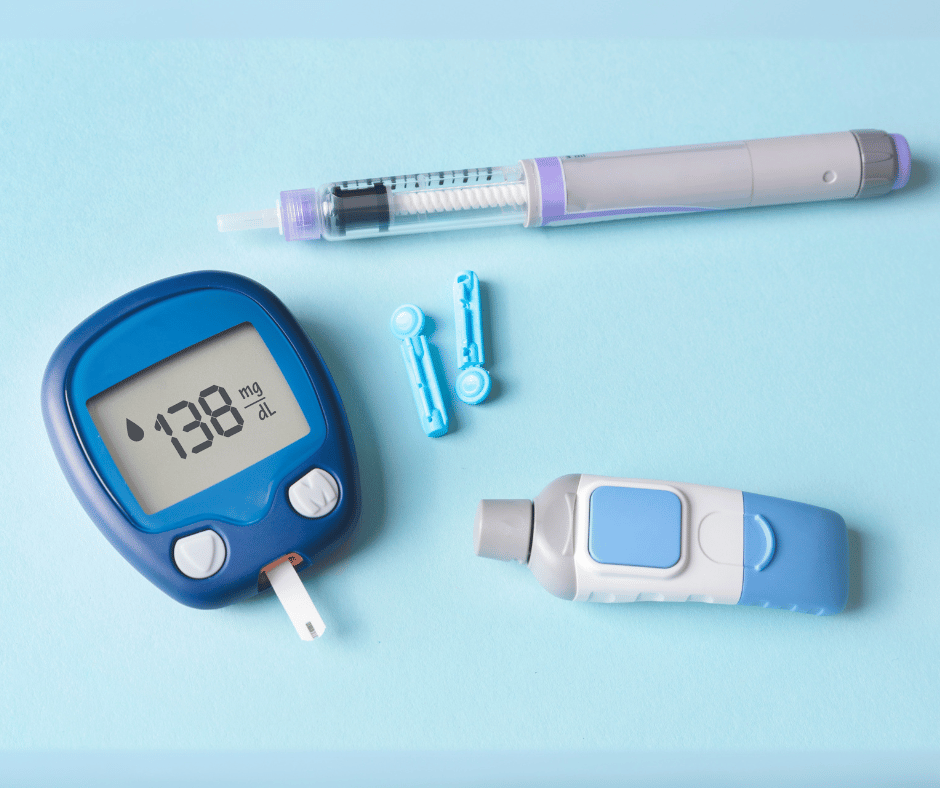
Glucometers are essential devices for individuals suffering from diabetes. Part of a diverse array of devices that help diabetic people to improve their quality of life, these compact devices provide quick and accurate readings, enabling users to make informed decisions about their diet, medication and overall health management.
This article will discuss the characteristics and applications of different glucometers, as well as offer guidance on their classification and grouping based on regulatory standards in Colombia.
Types of Glucometers
There exist two main types of glucometers:
- Standard Glucometers: these follow the elemental structure previously described, requiring manual entry of blood glucose readings. They are primarily designed for intermittent glucose blood sampling when frequent testing.
- Continuous Glucose Monitors (CGMs): while CGMs follow a similar elemental structure as standard glucometers, they incorporate additional elements to deliver a different kind of monitorization. These systems consist of a sensor placed under the skin to measure glucose levels continuously. The sensor sends data to a receiver or smartphone app (for wireless versions), providing real-time glucose readings, trends, and alerts for better management. This type of glucometer is designed for tight blood sugar control, people with an insulin pump, or with high and/or abrupt variations of blood glucose levels.
Classification and Grouping of Glucometers
Glucometers (either standard or CGMs) fall under Class IIa medical devices under Colombian regulatory framework.
It is also important to note that usually, other products considered as medical devices by themselves are often sold in form of a kit along with glucometers. The most common, being lancets for blood samples, and test strips. As medical devices, these examples have to be registered in separate sanitary registrations. A brief classification analysis of the mentioned devices is:
- Lancet devices & puncturing lancet devices: Class IIa
- Test strips: Class II
Once registered, new registrations of kits containing any combination of these previous devices can be put together as needed.
In our Classification and Grouping Ultimate Guide, we outline the necessary requirements for different models of medical devices to be registered together in a single registration. For the glucometers, the following criteria apply:
- Same device classification
- Same distinctive denomination (commercial name)
- Same intended use
- Same device technology (i.e., standard glucometers and CGMs need separate registrations)
- Same manufacturer
If you have any inquiries about the registration of glucometers and their grouping with different devices, or any other medical devices in the Colombian market, please feel free to contact us at contact@veraqueconsulting.com, or check our guidelines