As explained in the Colombia Classification and Grouping Guide, there is a comprehensive set of regulations for the categorization and grouping of medical devices (fully explained in the 1725 of 2005 decree, specifically in Article No. 5).
Using this regulatory framework as the legal basis, we will study in this article the classification and grouping of intraoral cameras for Colombia. We do provide a brief explanation of the product and how INVIMA considers the classification and grouping (particularly when different models need to be grouped).
General Characteristics of Intraoral Scanners
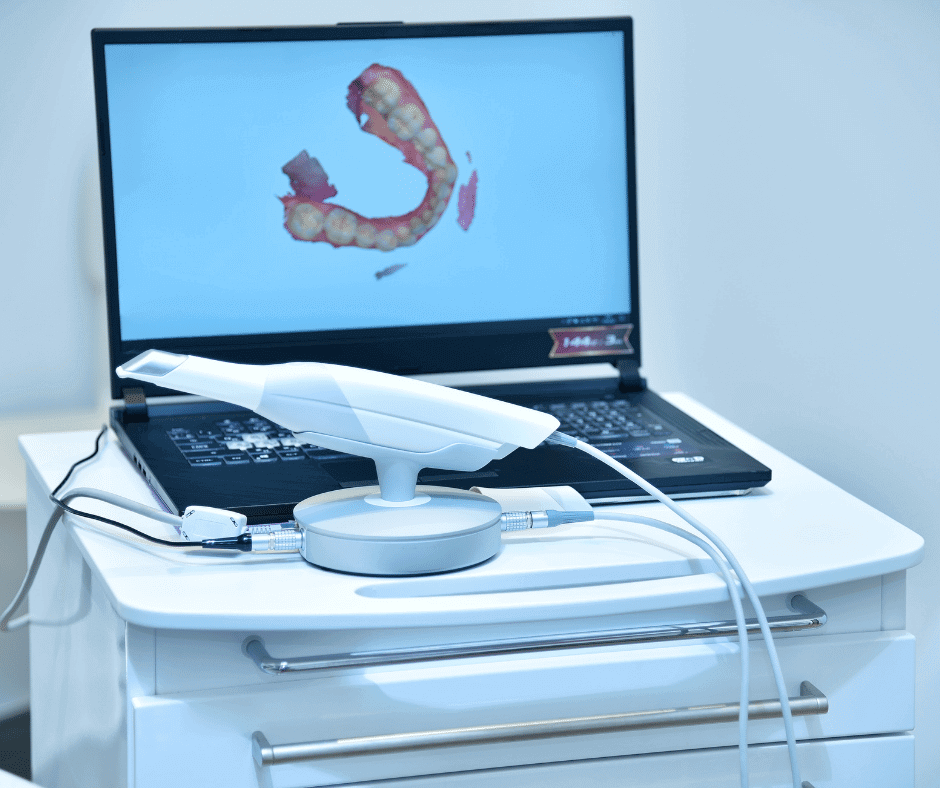
Also known as intraoral cameras, these products are specialized imaging devices used in the field of dentistry. They are employed to capture high-quality, detailed images and videos of a patient’s teeth, gums, and other oral structures; allowing dentists to examine areas that are not easily visible.
With the data provided from the camera, it is possible to evaluate cavities, gum diseases or oral lesions. The data can also be used to aid in treatment planning, tailored specifically for each patient.
Intraoral scanners are designed to be small, lightweight, and easy to maneuver inside the mouth. They typically consist of a handheld device with a camera at one end, which is often attached to a flexible or articulated hand piece or arm for better access to different areas of the mouth. These cameras are equipped with hi-resolution sensors that capture detailed images or videos of the oral cavity. The images are typically displayed in real-time on a computer monitor or a screen.
Classification According to the Level of Risk
Intraoral scanners fall under Class IIA medical devices, according to the Colombian regulatory framework, as they are active medical devices destined to be used for diagnostic purposes.
Grouping for Sanitary Registration Purposes
When attempting to group different medical devices into one sanitary registry, Article No. 28 of the Decree 4725 of 2005 must be considered. The following criteria should be taken into account:
- To group different references within the same registration, they must maintain consistency in:
- Device classification
- Intended use or indication
- Generic denomination
- Registration holder
- Manufacturer
- There may be differences in characteristics between different models if they do not modify the intended use or indication. (i.e., a wireless and a power cord/adapter variation of the same product could be grouped within the same registration).
- It is of particular interest that intraoral cameras are generally presented in different models, and it is common to group them in the same registration considering the mentioned points.
Conclusion
Oral cameras have become a valuable tool in the dentistry industry, enabling dentists to provide better patient care through improved diagnostics, treatment planning and patient education. This guideline intends to serve as a first approach when trying to define and categorize the device.
If you require further assistance in understanding any of the requirements discussed in this article, please contact us at contact@veraqueconsulting.com or check out our guidelines.