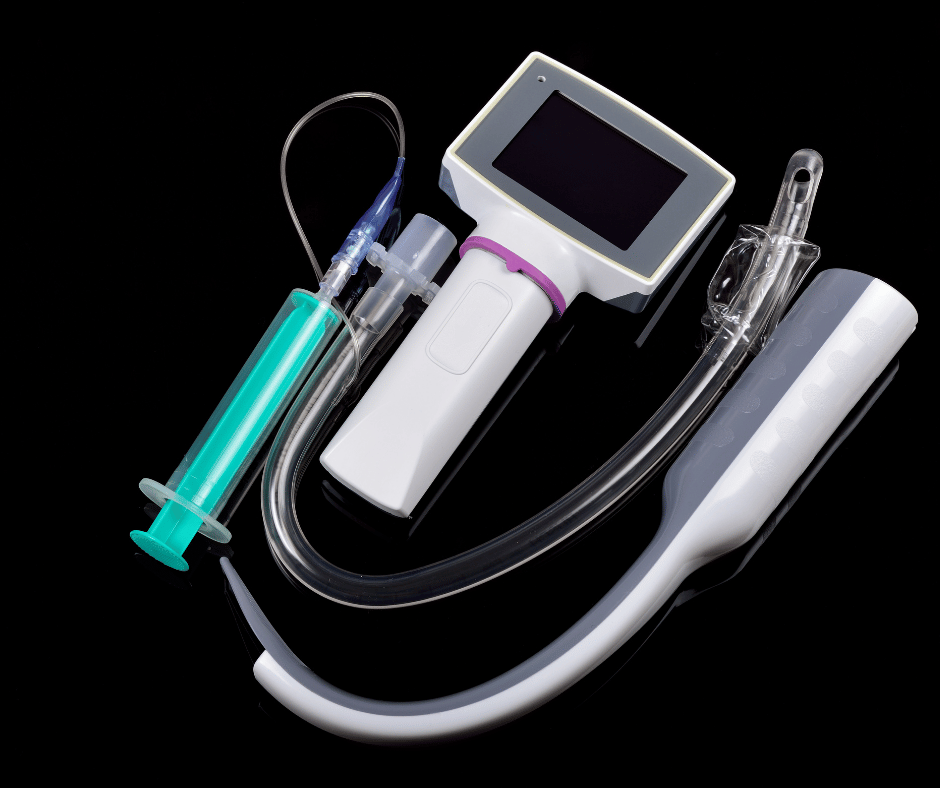
Laryngoscopes are essential medical devices used in anesthesiology and airway management. While traditional laryngoscopes have been in use for many years, advancements in technology have led to the development of video laryngoscopes. These innovative devices involve additional considerations for their sanitary requirements in Colombia.
Types of Laryngoscopes
- Classic or Conventional Laryngoscopes: consist of a handle, blade, and light source.
- Reusable: Typically, non-sterile and require sterilization after each use.
- Single-use: Sterile and discarded after use.
- Video Laryngoscopes: Feature a built-in camera at the blade’s tip. The camera, with the assistance of the light source, transmits real-time video images to a connected display.
- Components:
- Reusable: Handles and/or displays.
- Single-use: Blades (various sizes and shapes).
Classification in Colombia
Laryngoscopes are designed to facilitate visualization of the trachea for anesthesia or intubation. These devices are invasive but only used for brief periods (limited contact).
- Risk Profile Classification: Due to their potential risks, laryngoscopes fall under Class I or Class IIa .
Grouping in Colombia
- Conventional Laryngoscopes: The common presentations include:
- Single-piece: Handle and blade packaged together.
- Two-piece: Handle and blades sold separately.
- Kit: Handle with blades of various sizes and shapes, typically in a tray.
All these presentations can be included in a single registration as either a single device or grouped as a kit.
- Video Laryngoscopes: Typical presentations:
- Reusable and non-sterile: Handle and display or standalone display.
- Single-use and sterile: Blades in various sizes and shapes.
- Video laryngoscopes can be grouped into a single registration as a system based on the definition in Article 28 of Decree 4725 of 2005:
“Medical devices with the same risk classification, use and generic name that belong to the same owner and manufacturers (…) that are used together forming what can be called a system or kit, may be covered under a single sanitary registration according to the type of medical device”
Conclusion
Understanding the proper classification and grouping of laryngoscopes and video laryngoscopes is critical to get approvals to import and distribute those products in Colombia. Please contact us at contact@veraqueconsulting.com for professional guidance on how to successfully bring those products to Colombian market.