Classification and Grouping of Pacemakers in Costa Rica

Pacemakers have been considered an important precursor of many developments in science and cardiac medicine, playing a unique role in managing heart rhythm disorders. These medical devices can greatly improve the quality of life for individuals with heart rhythm disorders by reducing symptoms such as fatigue, dizziness, and fainting. They also help prevent serious complications […]
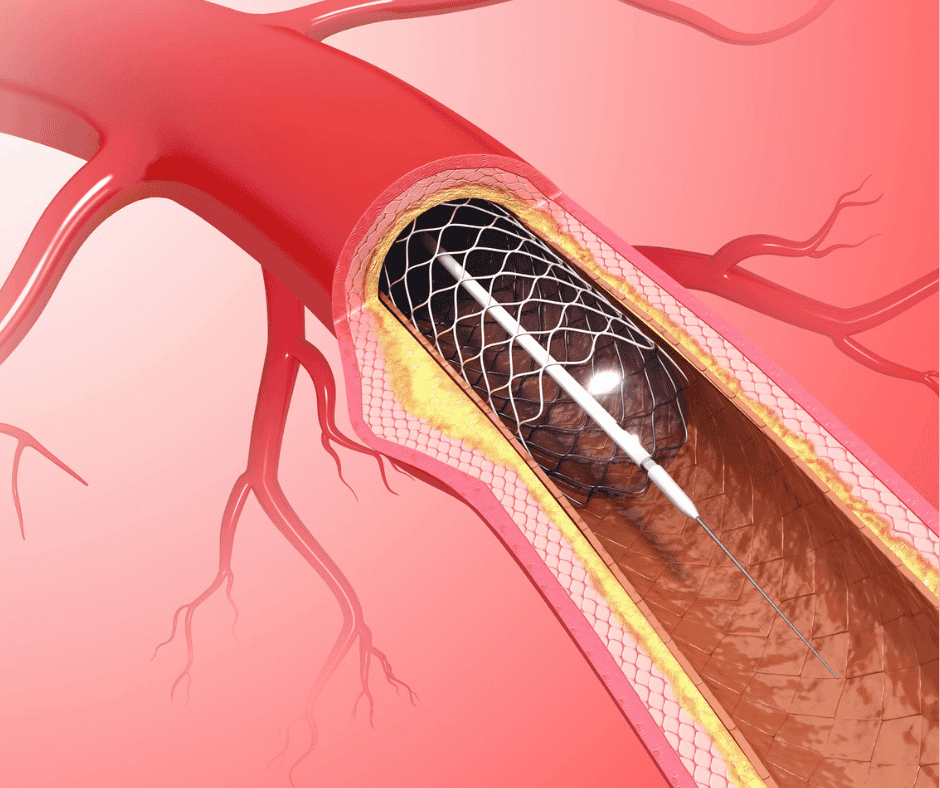
Classification and Grouping for Stents in Costa Rica

A stent is a small mesh tube that is placed inside a hollow structure in the body to keep it open. As mentioned in our Classification and Grouping Guide, there are a series of rules to consider when classifying and grouping medical devices in Costa Rica. In this article we will focus on explaining […]
Classification and Grouping of Balloon Catheters in Costa Rica
Balloon Catheters also known as dilatation catheters, stand as precise instruments in the array of medical devices. Employed to alleviate obstructions and stenosis across various bodily systems, they are a great exemplification of controlled intervention. In this article, we will discuss their structure, diverse applications, and existing types of Balloon Catheters. Additionally, we will […]
Classification and Grouping of Defibrillators in Costa Rica

Defibrillators are considered a critical tool, not only for medical practitioners, but for anyone attempting to save a life from a cardiac arrest episode. The use of defibrillators is a crucial part of the chain of survival in cases of sudden cardiac arrest. Early defibrillation, along with cardiopulmonary resuscitation (CPR), can significantly increase the chances […]
Classification and Grouping for Dialysis machines in Costa Rica
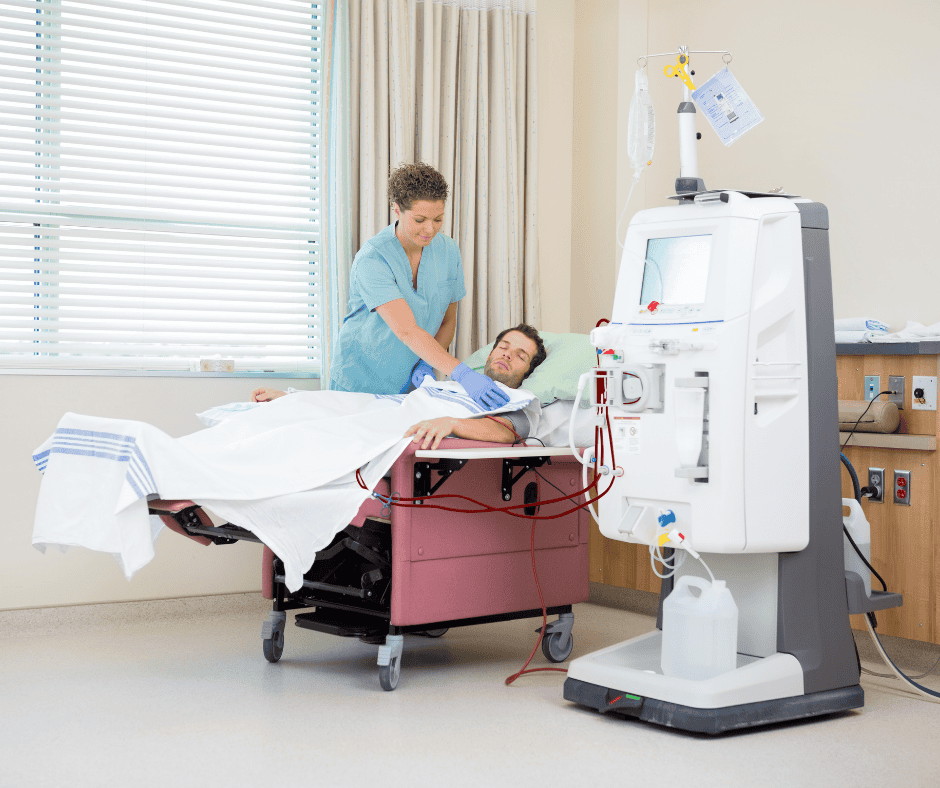
As mentioned in our Device Classification and Grouping Guide, there are a series of rules to consider when classifying and grouping medical devices. The above-mentioned rules are fully explained in Decree 43902-S. In this article we will focus on explaining strategies to classify and group a Dialysis machine and the common accessories related to […]
Classification and Grouping of Colposcopes in Costa Rica
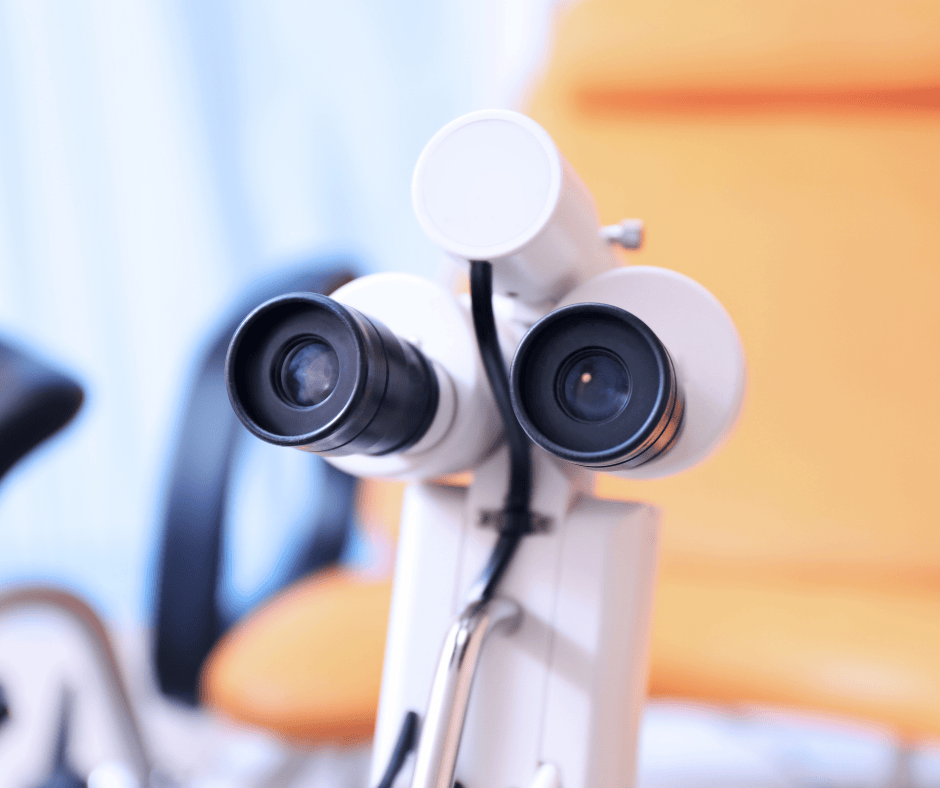
Colposcopes are indispensable tools in gynecological examinations, significantly contributing to the early detection and treatment of cervical and other genital abnormalities. This medical device is crucial for diagnosing various conditions, including cervical cancer and other abnormalities. In this article, we analyze the components and applications of colposcopes, as well as provide general criteria for their […]
Classification and Grouping of Hospital Sterilizers in Costa Rica

In hospitals and medical facilities, ensuring the cleanliness and sterility of medical instruments and equipment is essential to patient safety and infection control. By applying the method known as sterilization, equipment is rid of all forms of microbial life, including bacteria, viruses, fungi, and spores. In this article, we will focus on the characteristics, applications, […]
Class 1 low risk Medical Devices in Costa Rica

As mentioned in our Classification and Grouping Ultimate Guide, there are a series of rules to consider when classifying and grouping medical devices. The above-mentioned rules are fully explained in the decree 34482-S by the Ministry of Health of Costa Rica (MoH). In this article, we will enlist the medical devices that are found in […]
Applicable standards for Medical Devices

Medical devices are a broad group of products with different characteristics. Thus, many standards have been developed to establish general and particular specifications for them. In this text, we cover the most relevant international standards that can be found in the medical device industry that are useful for regulatory purposes. ISO 13485 Medical devices […]
Regístrelo, an on-line registration system in Costa Rica.

What is Regístrelo and how does it work? Paperwork and processes to register a medical device worldwide could be considered complicated. In addition, the timelines necessary to evaluate such documents might demotivate manufacturers around the globe. Fortunately, and recognizing the need to simplify and make the entire process more agile, Ministries of Health in […]
