As mentioned in our Classification and Grouping Ultimate Guide, there are a series of rules to consider when classifying and grouping medical devices. The above-mentioned rules are fully explained in the decree 4725 of 2005 by INVIMA.
In this article, we will focus on explaining strategies to classify and group surgical instruments following simple general rules. We also detail some particularities that can be found according to our everyday experience.
General characteristics of Surgical Instruments
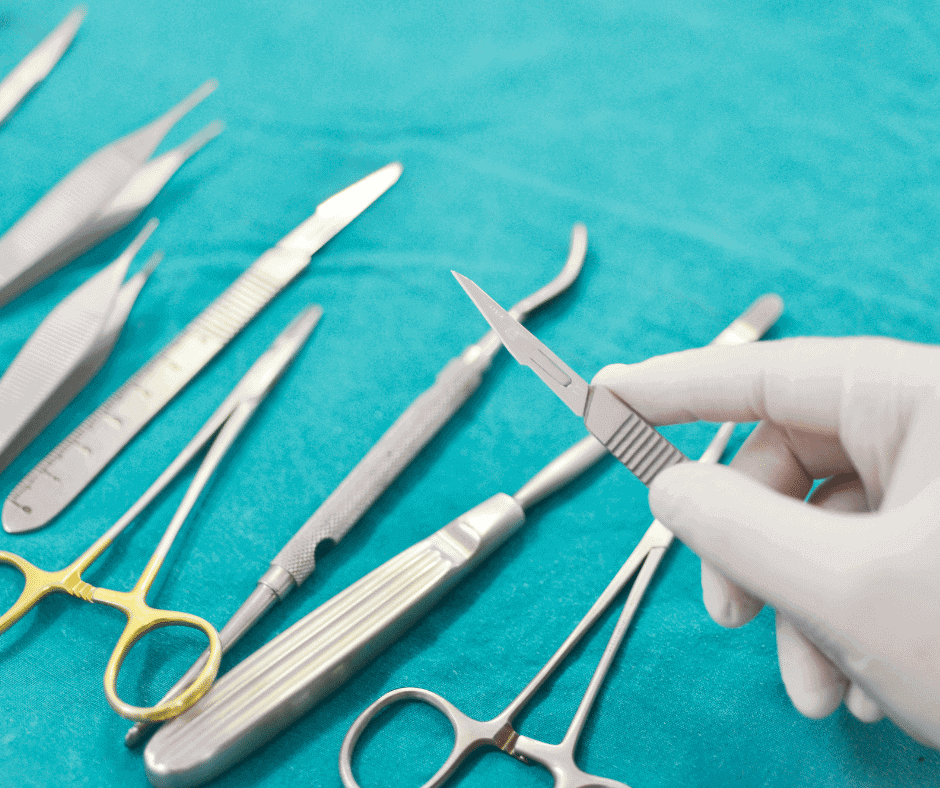
Talk about surgical instruments can embrace a wide range of specialties. Nevertheless, in this article we will focus on those common supplies used in the medical practice during a surgery (e.g. forceps, scalpels, clamps, retractors, and scissors).
Manufacturers of these instruments generally manage catalogues with hundreds of models that vary in size and intended use. We have seen for years, that a normal practice in the manufacturing cycle is to receive an unprocessed instrument (or the raw material) from one country and processed it in another. This particular case, when the instruments are processed in different sites, is a key point to identify the real manufacturer –a relevant entity in regulatory terms.
Every point described so far has regulatory implications that we will explain in the following sections.
Classification according to the level of risk
Surgical instruments by its nature are classified as Class I medical devices in Colombia. Nevertheless, those will be considered Class IIa if sold for single use.
Grouping for Sanitary Registration purposes
The classification for Surgical Instruments is pretty straightforward. Yet, it is still necessary to consider a few points regarding the grouping criteria. Specifically, surgical instruments can be grouped in the same sanitary registration if they have the same:
- Level of risk
- Use or indication
- Generic name
- Same holder
- Same manufacturer
As referred at the beginning of this article, the manufacturer is not necessarily an obvious entity to define. For instance, some countries consider the surgical instruments as low risk devices and exempt them of extensive review. Therefore, they get approval in industrialized countries (e.g. USA, Europe) but not in their country of origin. In Colombia, according to article 17 of the Decree 4725 of 2005, medical devices must have a sanitary registration, or a marketing approval issued by INVIMA, regardless of whether or not this has the sanitary approval from the country of origin.
Another challenge to consider is grouping thousands of products –a common scenario within the surgical instruments field. The best way to proceed in such cases is grouping the instruments based on its intended use. Nevertheless, we also understand that grouping scalpels in one registration, retractors in another and so on, ends up in a considerable number of registrations and therefore, in an economic investment to take in mind. Therefore, a riskier strategy if the number of projects is out of budget, is to comply with the criteria referred at the beginning of this section. In other words, as many similar characteristics the products share each other, the better the chances to group them.
As a side note, instruments for the orthopedic sector follows a standard presentation on which implants and instruments are sold in separate non-sterile trays. This makes the grouping of the instruments easier. You can check more details about it in this article.
Thanks for reading this article. If you have any question, feel free to contact us at contact@veraqueconsulting.com or check our guides.