Classification and Grouping for Ultrasound Systems In Colombia
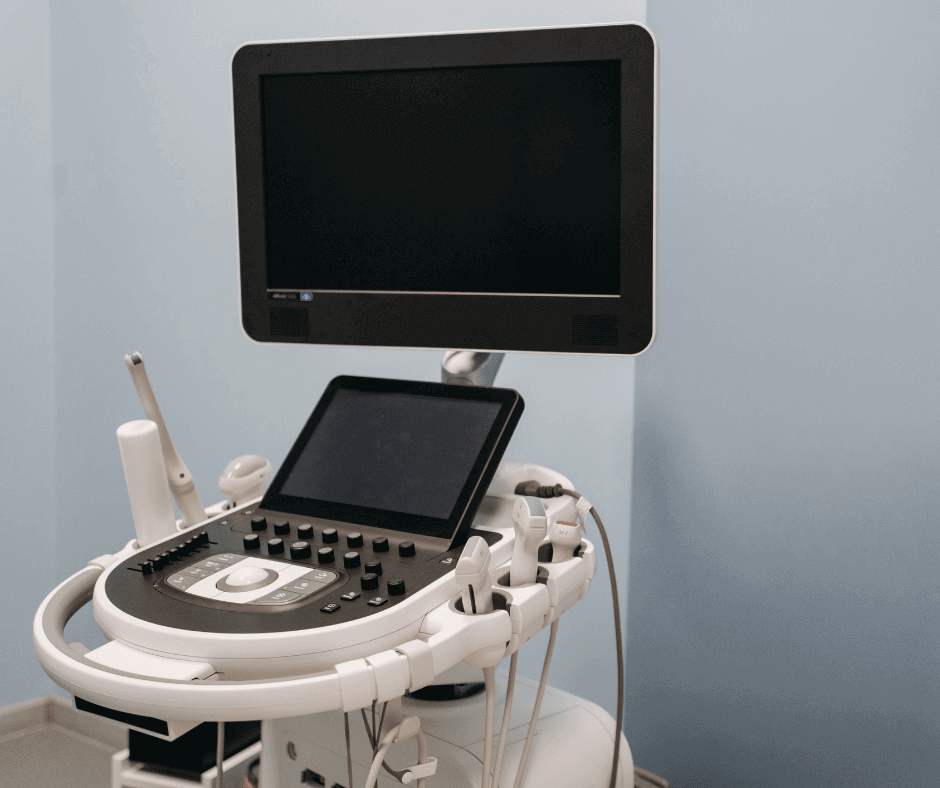
As mentioned in our Classification and Grouping Guide, there are a series of rules to consider when classifying and grouping medical devices. The above-mentioned rules are fully explained in Article No. 5 of the decree 4725 of 2005. In this publication, we will focus on explaining strategies to classify and group an ultrasound equipment […]
Classification for In-Vitro Diagnostics in Colombia

As mentioned in our Classification and Grouping Ultimate Guide, there are a series of rules to consider when classifying and grouping In Vitro Diagnostic devices in Colombia. Such rules are fully explained in the decree 3770 of 2004 by INVIMA. In this article, we will focus on explaining strategies to classify and group In-Vitro Diagnostic […]
Classification and Grouping for Orthopedic Implants in Colombia
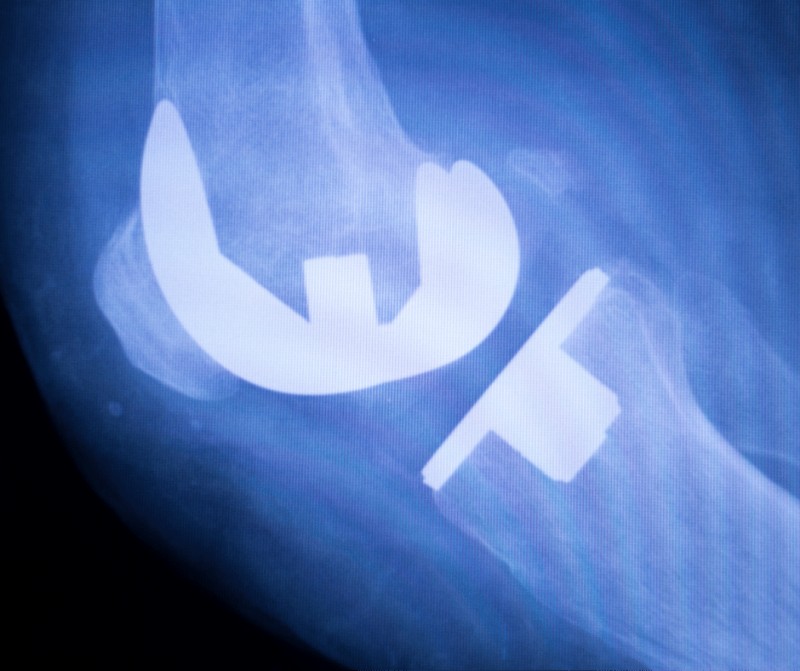
As mentioned in our Classification and Grouping Ultimate Guide for Colombia, there are a series of rules to consider when classifying and grouping medical devices. The above-mentioned rules are fully explained in the decree 4725 of 2005 by INVIMA. In this article, we will focus on explaining strategies to classify and group orthopedic implants following […]
Applicable standards for Medical Devices

Medical devices are a broad group of products with different characteristics. Thus, many standards have been developed to establish general and particular specifications for them. In this text, we cover the most relevant international standards that can be found in the medical device industry that are useful for regulatory purposes. ISO 13485 Medical devices […]
