Is this a real opportunity for those Medical Device manufacturers to participate without a sanitary registration?
As published by the UNOPS (United Nations Office for Project Services) the last 25 of August 2020, the Mexican Public Healthcare system signed an agreement to promote an international tender binding for all those companies interested to sell medical supplies in Mexico (medical devices included). This is for the period 2021-2024. In this article, we would like to explain some of the reasons behind this bid and opportunities you may find interesting.
During this year, the Mexican Government expressed the necessity to acquire low prices medical supplies with the support of international entities. This was legally approved in the official diary the last August 11. Such amendment, basically allows the Mexican Government to acquire any product without following the rules that used to be the legal basis for every public bid performed in Mexico.
A few days after such legal amendment, the Government worked with the UNOPS to publish the procurement of medicines and medical aid supplies (medical devices) in Mexico. These are a few key facts to consider:
- Published: 25-Aug-2020
- Deadline: 15-Sep-2020 23:59 (GMT -5.00) Central Time (US & Canada), Mexico City
- 900 type of medical devices required
In addition, it was also reported that:
- Participants do not necessarily require to present sanitary registrations approved in Mexico by COFEPRIS
- It is expected that for 2021 the value of this bid would be approximately $6 billion USD
It is worth mentioning that this is the first time the Mexican Government makes such acquisition process. And this is important to the country, considering that the public sector covers 70-80% of the total medical services.
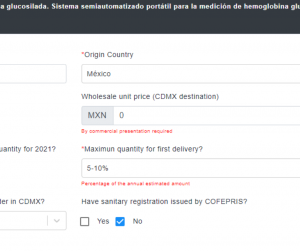
Even though a COFEPRIS sanitary registration is not a requirement to participate, we found in the official UNOPS platform that once you submit your application, they asked you if a COFEPRIS sanitary registration has been granted. This does not necessarily implicates that you need such registration.
From UNOPS webpage (taken on August 9th 2020)
In conclusion, there is still uncertainty if this process will effectively promotes a real benefit on prices. Nevertheless, we consider that if you are a medical device manufacturer with local sanitary approvals in your country, this could be a great chance to get into the Mexican market.
In case you are interested to know more about this bid, please send us an email to contact@veraqueconsulting.com