Classification and Grouping of Anesthesia Circuits in Mexico
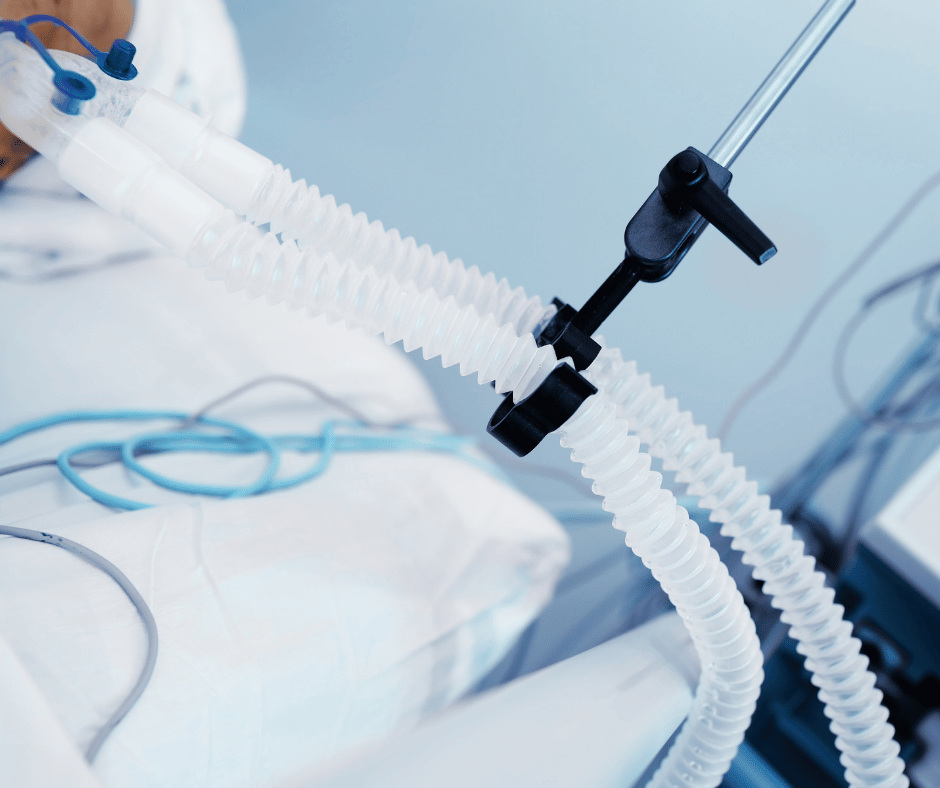
This article continues our series on anesthesia-related medical devices. Having previously covered anesthesia masks, endotracheal tubes, and laryngeal masks, we now turn our attention to the regulatory process for registering anesthesia circuits in Mexico. What are Anesthesia Circuits? Anesthesia circuits, also known as anesthesia breathing systems, are key medical devices used in anesthesia […]
Classification and Grouping for Endotracheal Tubes in Mexico
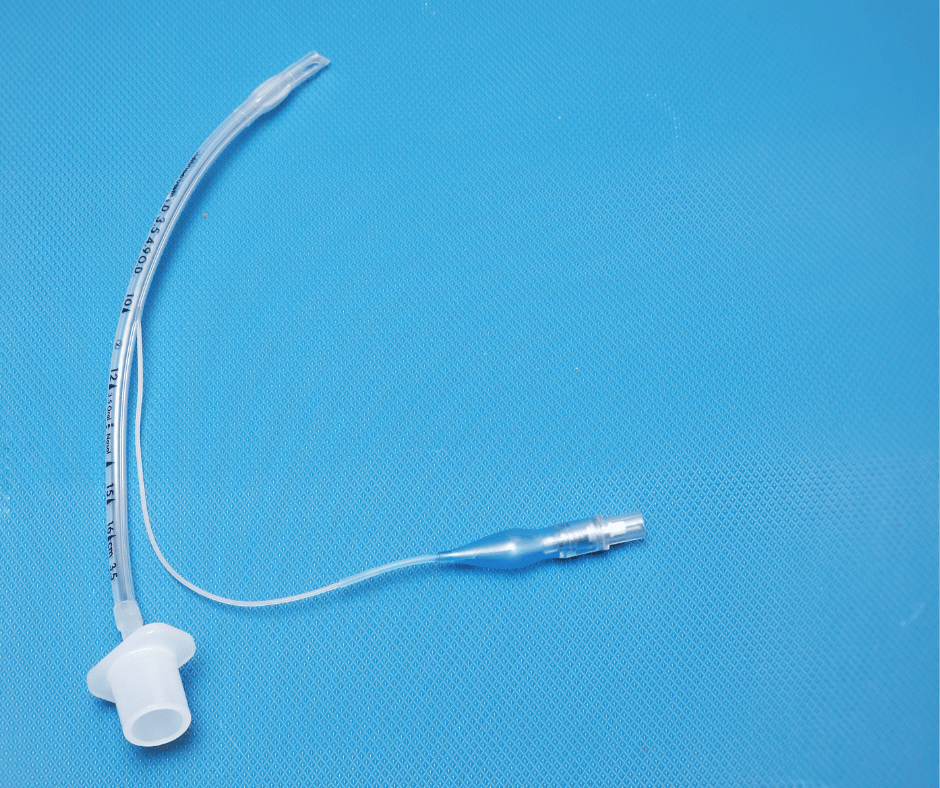
This article aims to provide a clear understanding of the classification and grouping for the sanitary registration process of Endotracheal Tubes (ETTs) and Laryngeal Mask Airways (LMAs) in the Mexican market. Like anesthesia masks, these medical devices are crucial for airway management during anesthesia and respiratory emergencies. ETTs ETTs are flexible tubes inserted into […]
Classification and Grouping for Anesthesia and Oxygen Delivery Masks in Mexico
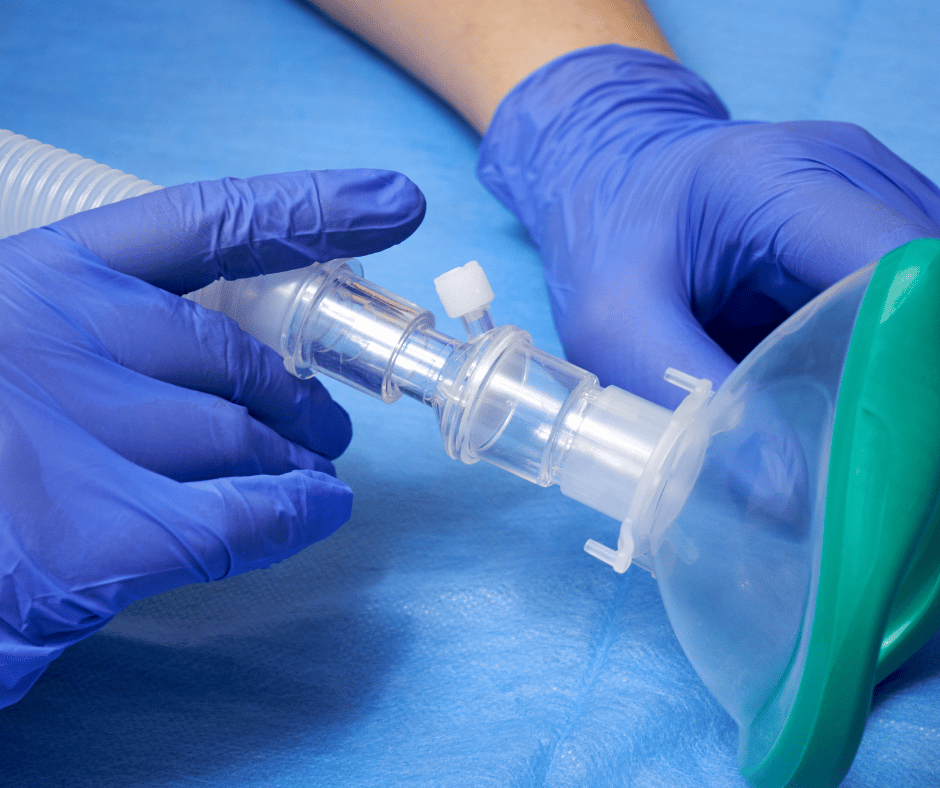
Face masks are considered basic medical devices used in various healthcare settings, from intensive care units to emergency rooms. Their primary function is to deliver a controlled mixture of gases, such as oxygen, air, or anesthetic agents to the patient. Given the diverse range of masks available and their specific applications, understanding their classification and […]
Classification and Grouping for Laryngoscopes and Video Laryngoscopes (Mexico)
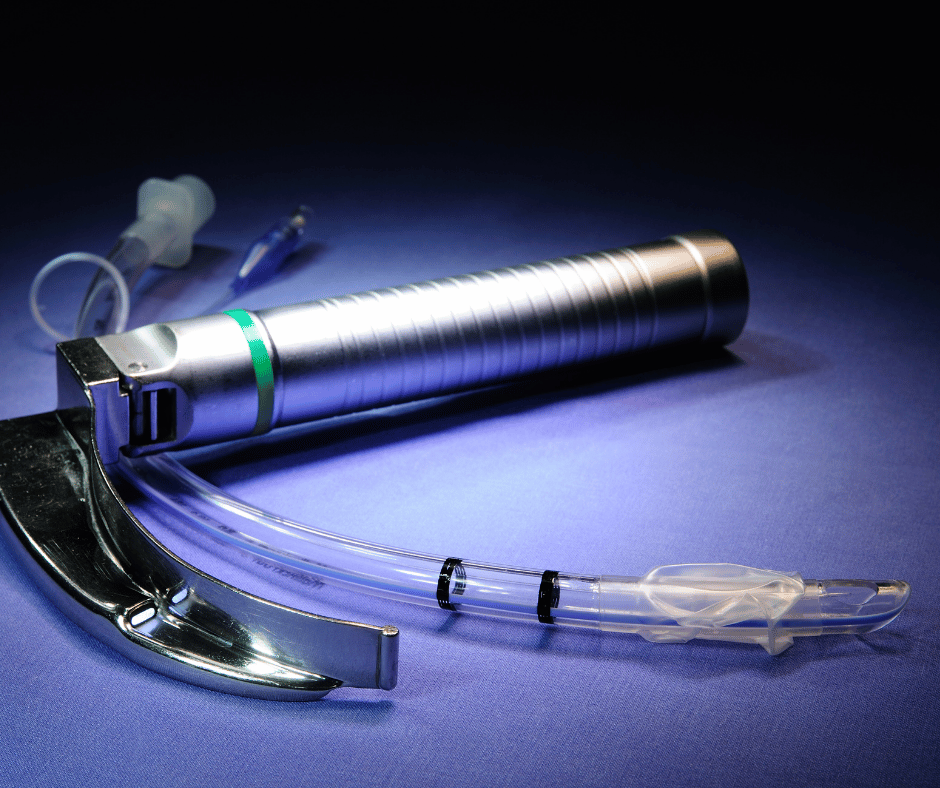
Laryngoscopes are essential medical devices used in anesthesiology and airway management. While traditional laryngoscopes have been in use for many years, advancements in technology have led to the development of video laryngoscopes. These innovative devices involve additional considerations for their sanitary requirements in Mexico. Types of Laryngoscopes Classic or Conventional Laryngoscopes: consist of […]
Hygienic Products as Medical Devices in Mexico

Hygienic products, typically thought of as substances for personal cleanliness, hold a unique position in Mexico’s regulatory landscape. Classified as medical devices, these products fall under the scope of COFEPRIS, requiring sanitary registration to be commercialized in the country. In this article, we briefly describe the characteristics and regulatory requirements surrounding these products. Defining […]
Clinical Evaluation Studies for Medical Device Approval in Mexico

Clinical evaluation for medical devices is critical because it provides the scientific evidence to demonstrate that devices are safe and effective for use in real-world situations. For sanitary registration purposes in Mexico, is acceptable to submit clinical trials performed in this country or abroad. However, there are other ways to fulfill the clinical evaluation requirements. […]
Classification and Grouping of Liposuction Equipment in Mexico
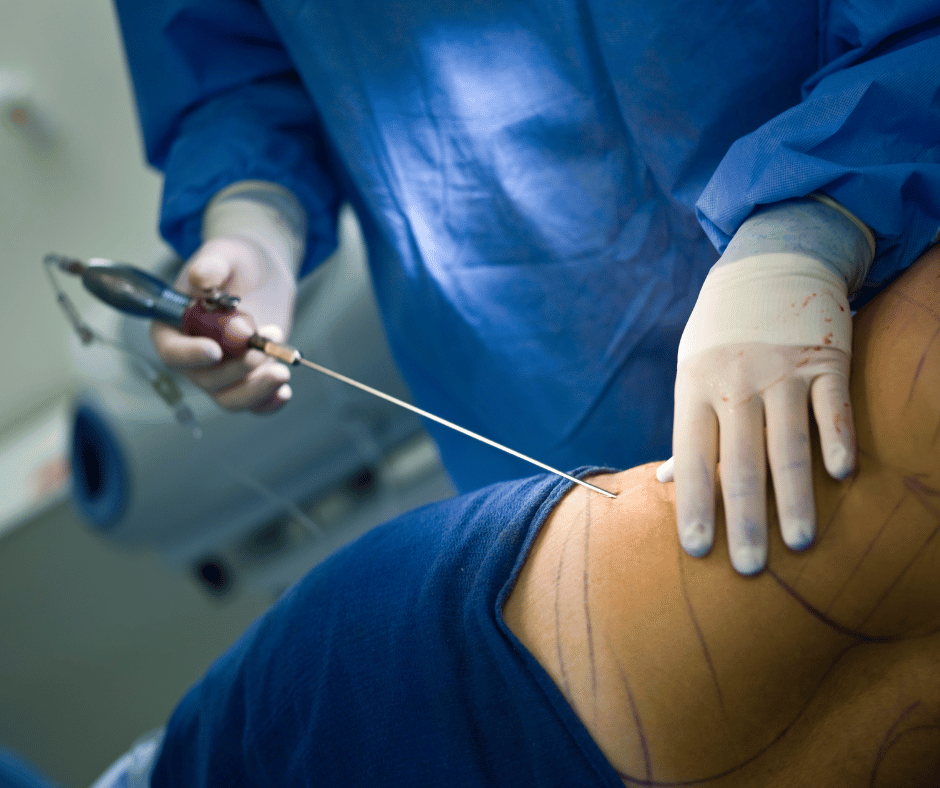
Liposuction, also known as lipoplasty, is a surgical procedure designed to remove excess fat deposits from specific areas of the body, such as the abdomen, hips, thighs, buttocks, arms, and neck. This procedure not only enhances body contour but also contributes to overall aesthetic improvement. The success and safety of liposuction largely depends on the […]
Immediate Service Counter – Rights Transfer Update

The Immediate Service Counter is a measure implemented by The Federal Commission for the Protection against Sanitary Risks (COFEPRIS) to improve and simplify submission processes regarding sanitary registrations. An important update on its scope has been enacted to include rights transfers of sanitary registrations, aimed at enhancing the quick-response services offered. In this article, […]
Instructions for Use and User Manuals for Medical Devices in Mexico

Instructions for Use (IFU) and user manuals are essential documents for any medical device. They ensure the safe and effective use of the device by providing clear, step-by-step guidance to healthcare professionals and/or patients. While these documents are standardized to a certain extent, there are specific requirements to keep in mind when registering such products […]
Understanding COFEPRIS Fees for Medical Device Registrations in Mexico

Entering the Mexican medical device market requires planning regulatory approvals and associated costs. A key aspect is understanding the fees charged by COFEPRIS and how the Marketing Authorization Holder (MHA) must cover them. This article breaks down those topics and outline important considerations for 2024. Legal Basis for Fees: COFEPRIS fees are established in […]
