Immediate Service Counter – Rights Transfer Update

The Immediate Service Counter is a measure implemented by The Federal Commission for the Protection against Sanitary Risks (COFEPRIS) to improve and simplify submission processes regarding sanitary registrations. An important update on its scope has been enacted to include rights transfers of sanitary registrations, aimed at enhancing the quick-response services offered. In this article, […]
Instructions for Use and User Manuals for Medical Devices in Mexico

Instructions for Use (IFU) and user manuals are essential documents for any medical device. They ensure the safe and effective use of the device by providing clear, step-by-step guidance to healthcare professionals and/or patients. While these documents are standardized to a certain extent, there are specific requirements to keep in mind when registering such products […]
Understanding COFEPRIS Fees for Medical Device Registrations in Mexico

Entering the Mexican medical device market requires planning regulatory approvals and associated costs. A key aspect is understanding the fees charged by COFEPRIS and how the Marketing Authorization Holder (MHA) must cover them. This article breaks down those topics and outline important considerations for 2024. Legal Basis for Fees: COFEPRIS fees are established in […]
Colposcopes Classification and Grouping in Mexico

Colposcopes are indispensable tools in gynecological examinations, significantly contributing to the early detection and treatment of cervical and other genital abnormalities. This medical device is crucial for diagnosing various conditions, including cervical cancer and other abnormalities. In this article, we analyze the components and applications of colposcopes, as well as provide general criteria for their […]
Medical Device Biocompatibility in Mexico: From ISO 10993 to COFEPRIS Requirements

As we have explored in a previous article about medical device standards, this medical device industry is extensive, and the regulations are just as vast. This time, we are diving a bit deeper into a crucial topic: Biocompatibility Studies. Here we will talk about ISO 10993, the standard for evaluating biocompatibility for medical devices and […]
Classification and Grouping of Endoscopes in Mexico
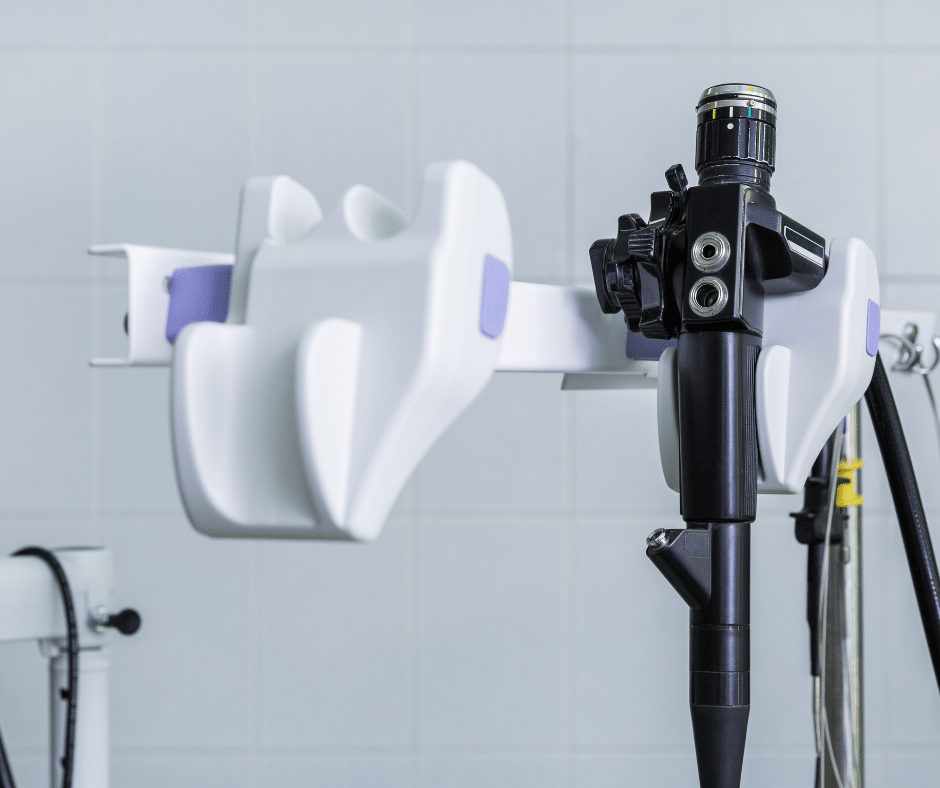
Endoscopes are indispensable tools in modern medicine, allowing healthcare professionals to diagnose and treat conditions within the body with minimal invasiveness. With the recent advancements in medical technology, the range of endoscopic equipment has expanded significantly. This article provides an overview of the types of endoscopes, their applications, and the regulatory requirements for their classification […]
Classification and Grouping of Hospital Sterilizers in Mexico

In hospitals and medical facilities, ensuring the cleanliness and sterility of medical instruments and equipment is essential to patient safety and infection control. By applying the method known as sterilization, equipment is rid of all forms of microbial life, including bacteria, viruses, fungi, and spores. In this article, we will focus on the characteristics and […]
Pacemakers Classification and Grouping in Mexico
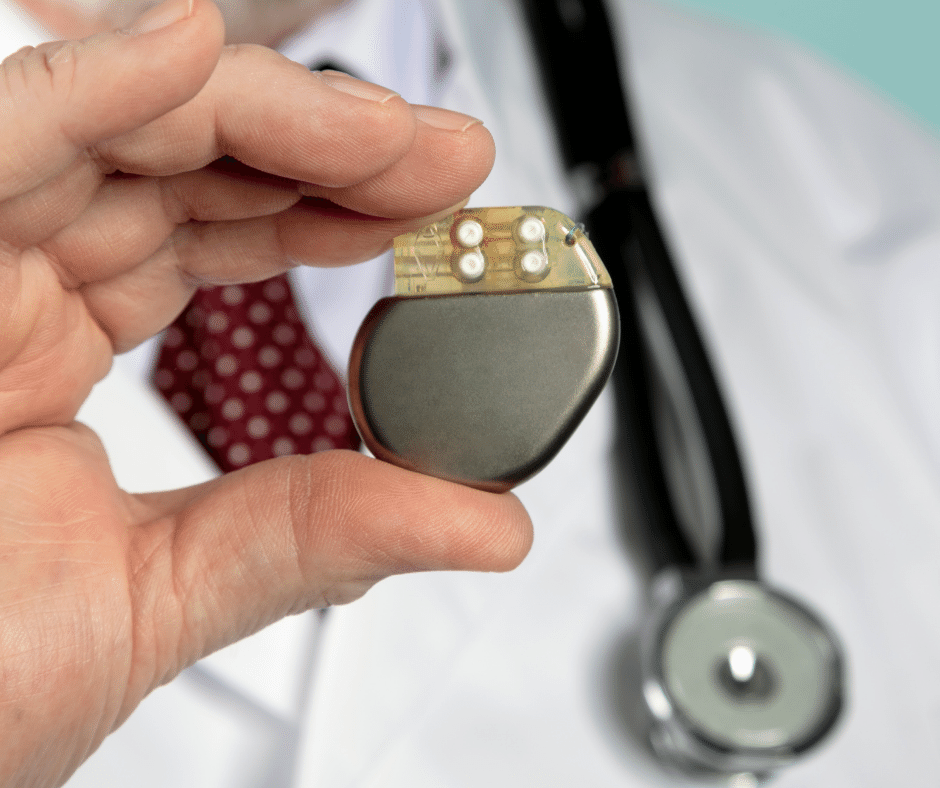
The pacemaker has been considered an important precursor of many developments in science and cardiac medicine, playing a unique role in managing heart rhythm disorders. Pacemakers can greatly improve the quality of life for individuals with heart rhythm disorders by reducing symptoms such as fatigue, dizziness, and fainting. They also help prevent serious complications associated […]
Defibrillators Classification and Grouping in Mexico

Defibrillators are considered a critical tool, not only for medical practitioners, but for anyone attempting to save a life from a cardiac arrest episode. The use of defibrillators is a crucial part of the chain of survival in cases of sudden cardiac arrest. Early defibrillation, along with cardiopulmonary resuscitation (CPR), can significantly increase the chances […]
Classification and Grouping of Balloon Catheters in Mexico
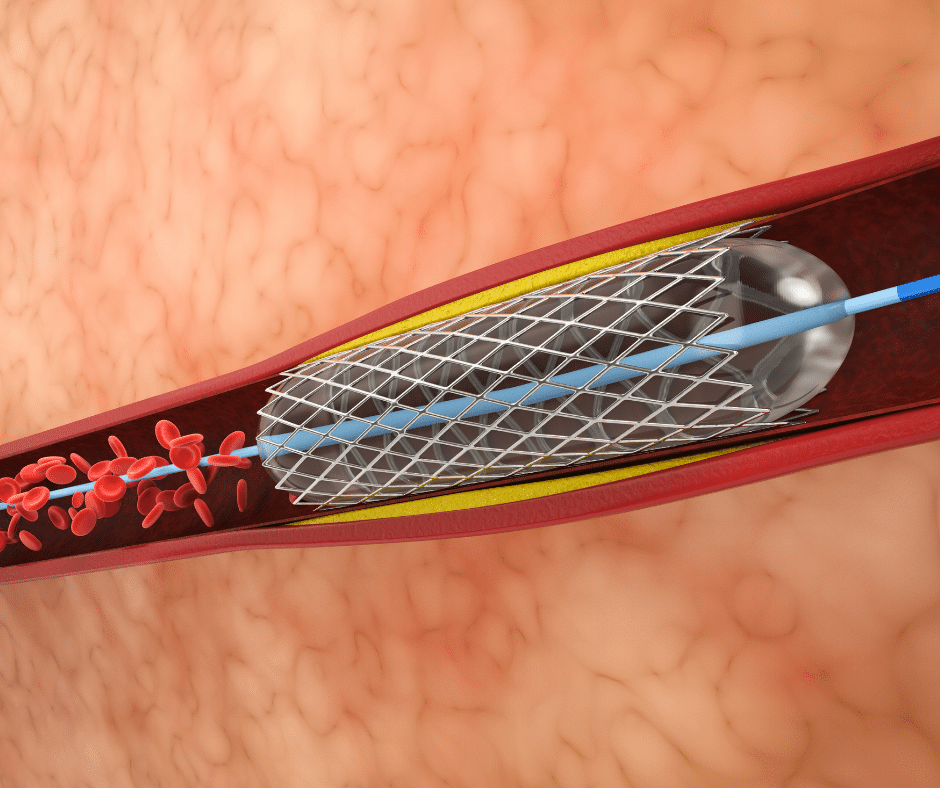
Balloon Catheters stand as precise instruments in the array of medical devices. Employed to alleviate obstructions and stenosis across various bodily systems, they are a great exemplification of controlled intervention. In this article, we will discuss the structure, diverse applications, and existing types of Balloon Catheters. Additionally, we will review general guidelines on their classification […]
