Classification and Grouping of Bone Substitutes in Mexico
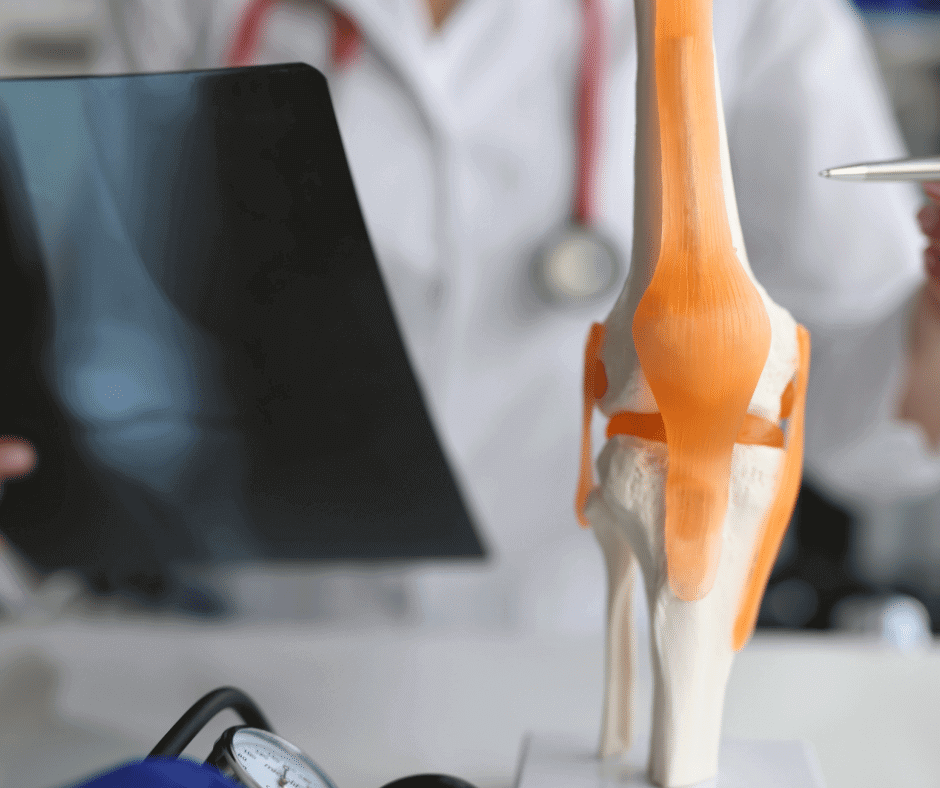
Bone substitutes, considered as medical devices, offer a wide range of products. Emerging from the necessity for treating a vital component of a patient’s body, the landscape of bone substitute materials and their applications has expanded to meet the growing demands of healthcare. In this article, we discuss different types of bone substitutes, as […]
Classification and Grouping of Laser Medical Devices in Mexico

The incorporation of laser technology to medical devices has come to play a crucial role in a diverse range of healthcare applications. Beyond their association with cosmetic procedures, many medical devices include lasers, transforming patient care and attention. From breaking down kidney stones to corneal correction, laser technology enhances healthcare and continues to evolve as […]
Classification and Grouping of Patient Monitoring Systems in Mexico
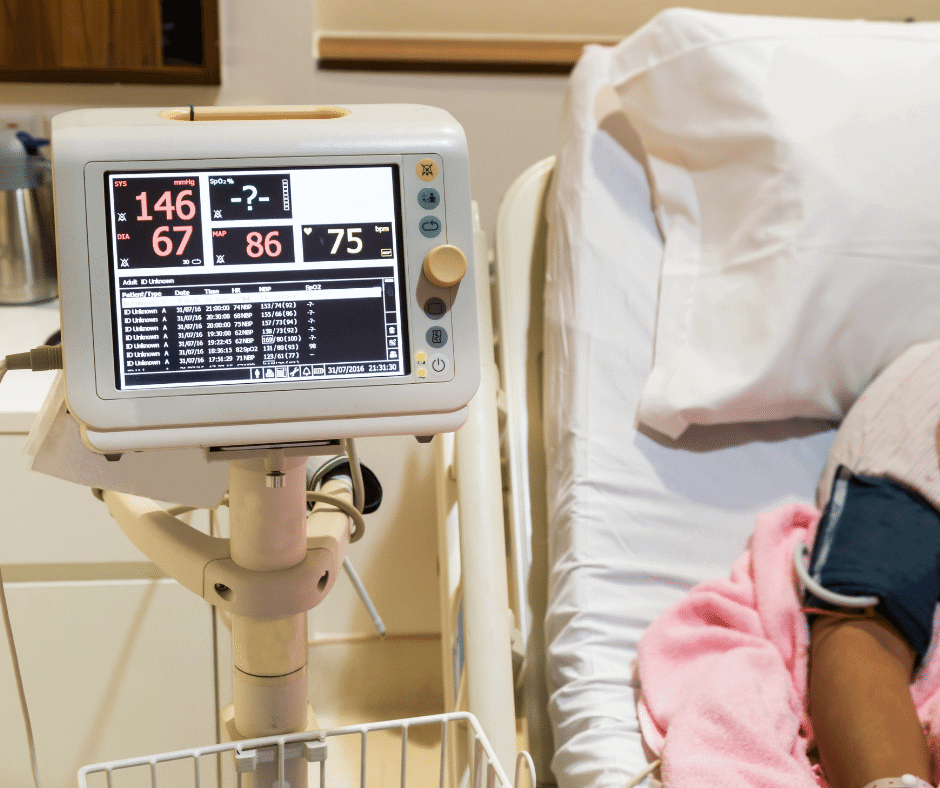
Patient monitoring systems nowadays offer an ample array of healthcare solutions. From clinical healthcare to personal use, healthcare monitors evolve to satisfy new needs, and so does their basic structure and components. This continuous state of evolution often constitutes a challenge when trying to classify and group them in the context of the Mexican regulatory […]
Classification and grouping of Mouthwashes as a Medical Device in Mexico

As discussed in our Classification and Grouping Ultimate Guide, the first step on classifying a product is to determine if it qualifies as a medical device in Mexico. A particular case is when products are considered Over the Counter Drugs or require a different type of licensing in other regions, but may fall under the medical […]
Classification of Software as a Medical Device in Mexico

As previously discussed in our article for Software as a Medical Device (SaMD), a significant development has occurred in the Mexican regulatory framework. The introduction of the first official approach to regulating SaMD marks a crucial step in the right direction, aiming to establish a faster and more comprehensive regulatory environment. However, the rapid […]
Authorization for the Acquisition and Transfer of Ionized Radiation Sources in Mexico

In Mexico, when registering medical devices related to Ionized Radiation Sources, there is a special subset of documents to be presented. The Authorization for the Acquisition and Transfer of Ionized Radiation Sources is one of these requirements. In this article, we provide a brief overview of this document within the context of the Mexican […]
Certificate to Foreign Government for Device Not Exported from the United States

As mentioned in our article about the Equivalency Agreement and our Ultimate Guide for Regulatory Affairs in Mexico, one of the choices you have to register a Medical Device for its sale and distribution in Mexico is the Equivalency Agreement route. In this regulatory pathway, one of the key documents for the FDA Equivalency route […]
Electrical Testing Standards for medical electrical equipment and its use in Mexico
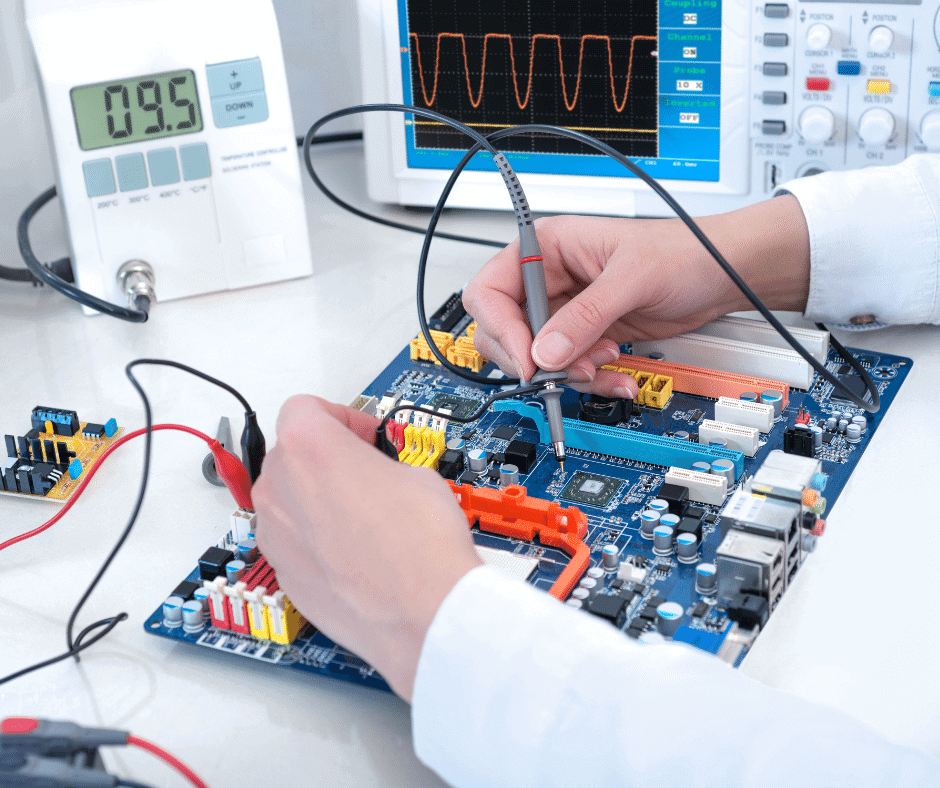
In a previous article, we have reviewed the regulations for medical electrical equipment (MEE) in Mexico and mentioned the cases when a medical electrical equipment needs to meet local standards. In this article we will review the applicability of a key series of international technical standards for medical electrical equipment, the IEC 60601. […]
Classification and Grouping for Urology Medical Devices in Mexico
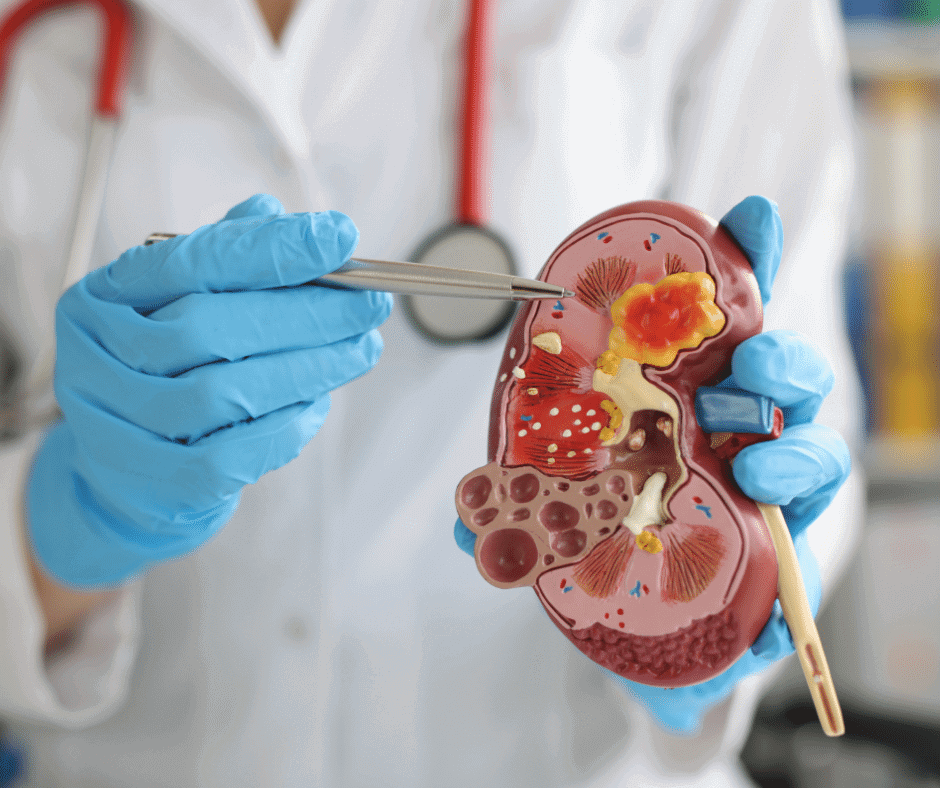
When registering Medical Devices in Mexico, the classification and grouping strategy is a key activity for optimizing the number of registrations (based on 23 rules). You can find some examples of the classification and grouping criteria in our previous articles for: Orthopedic Implants Ultrasound Systems Medical Imaging In vitro Diagnostics Surgical instruments Though, […]
Classification and Grouping for Dental Resins in Mexico

In our Classification and Grouping Ultimate Guide, we have explained how to classify and group medical devices according to the 23 rules detailed in the Appendix II of the Supplement of medical devices of the Mexican Pharmacopeia (FEUM). In addition, we have also explained how to make use of those rules to classify and group specific medical […]
