Instructions for Use and User Manuals for Medical Devices in Mexico

Instructions for Use (IFU) and user manuals are essential documents for any medical device. They ensure the safe and effective use of the device by providing clear, step-by-step guidance to healthcare professionals and/or patients. While these documents are standardized to a certain extent, there are specific requirements to keep in mind when registering such products […]
Understanding COFEPRIS Fees for Medical Device Registrations in Mexico

Entering the Mexican medical device market requires planning regulatory approvals and associated costs. A key aspect is understanding the fees charged by COFEPRIS and how the Marketing Authorization Holder (MHA) must cover them. This article breaks down those topics and outline important considerations for 2024. Legal Basis for Fees: COFEPRIS fees are established in […]
Colposcopes Classification and Grouping in Mexico

Colposcopes are indispensable tools in gynecological examinations, significantly contributing to the early detection and treatment of cervical and other genital abnormalities. This medical device is crucial for diagnosing various conditions, including cervical cancer and other abnormalities. In this article, we analyze the components and applications of colposcopes, as well as provide general criteria for their […]
Medical Device Biocompatibility in Mexico: From ISO 10993 to COFEPRIS Requirements

As we have explored in a previous article about medical device standards, this medical device industry is extensive, and the regulations are just as vast. This time, we are diving a bit deeper into a crucial topic: Biocompatibility Studies. Here we will talk about ISO 10993, the standard for evaluating biocompatibility for medical devices and […]
Classification and Grouping of Glucometers in Mexico

Glucometers are essential devices for individuals suffering from diabetes. Part of a diverse array of devices that help diabetic persons to improve their quality of life, these compact devices provide quick and accurate readings, enabling users to make informed decisions about their diet, medication and overall health management. This article will discuss the characteristics […]
Classification and Grouping of Balloon Catheters in Mexico
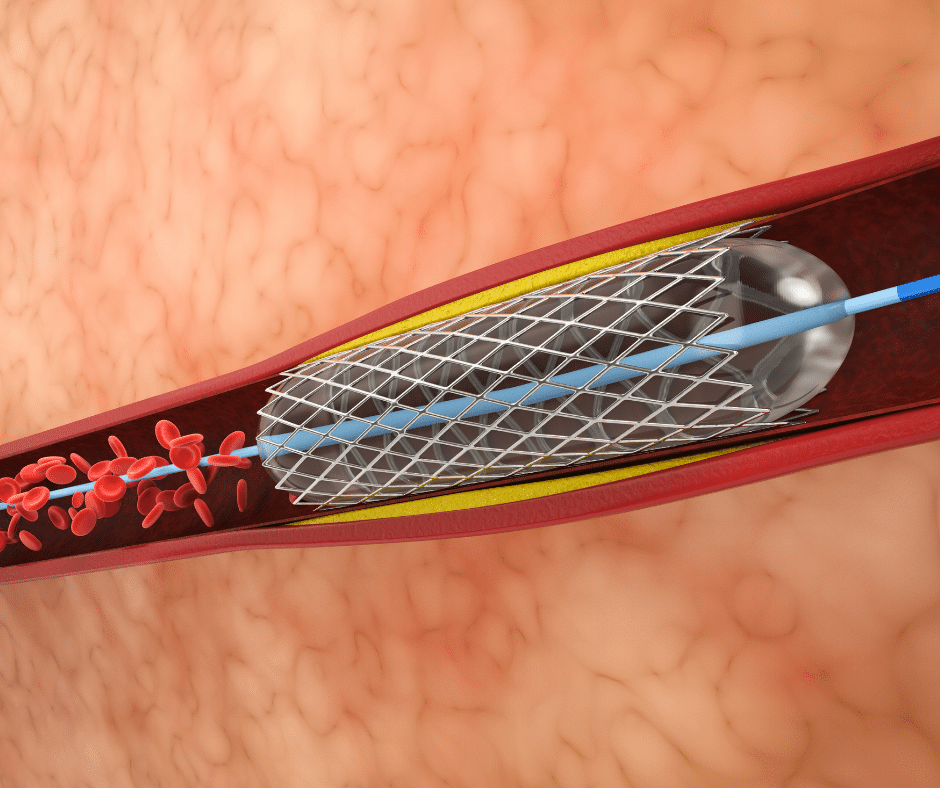
Balloon Catheters stand as precise instruments in the array of medical devices. Employed to alleviate obstructions and stenosis across various bodily systems, they are a great exemplification of controlled intervention. In this article, we will discuss the structure, diverse applications, and existing types of Balloon Catheters. Additionally, we will review general guidelines on their classification […]
Technovigilance for Medical Devices in Mexico

Introduction Technovigilance or Medical Device Surveillance is the activity that guarantees that any medical technology in Mexico is correctly working and describes how to report any incident related to the device. In this text, we summarize the technovigilance requirements in Mexico. Specifically: How to identify an adverse incident, When to report and not report […]
About the UNOPS international tender binding for Mexico

Is this a real opportunity for those Medical Device manufacturers to participate without a sanitary registration? As published by the UNOPS (United Nations Office for Project Services) the last 25 of August 2020, the Mexican Public Healthcare system signed an agreement to promote an international tender binding for all those companies interested to sell […]
Updates about COFEPRIS strategies to become digital
In the last months, COFEPRIS has been implementing different activities to digitalize new procedures focusing on a paperless policy. In this article, we briefly describe the expected digitalization transfer and how the regulatory process in Mexico may be impacted. COFEPRIS has initiated an ambitious project to digitalize the regulatory processes in a similar way […]
COFEPRIS legal structure changes

As we have previously explained in our Guide and Article, COFEPRIS is the regulatory arm of the Ministry of Health in Mexico which regulates the manufacturing, importation, marketing, and other activities related with health supplies including medical devices. Up to August 19, 2020 according to the Health Law, COFEPRIS was a separated administrative commission […]
